Abstract
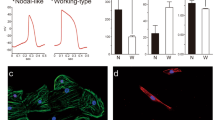
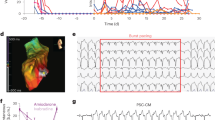
Ventricular tachyarrhythmias are the main cause of sudden death in patients after myocardial infarction. Here we show that transplantation of embryonic cardiomyocytes (eCMs) in myocardial infarcts protects against the induction of ventricular tachycardia (VT) in mice. Engraftment of eCMs, but not skeletal myoblasts (SMs), bone marrow cells or cardiac myofibroblasts, markedly decreased the incidence of VT induced by in vivo pacing. eCM engraftment results in improved electrical coupling between the surrounding myocardium and the infarct region, and Ca2+ signals from engrafted eCMs expressing a genetically encoded Ca2+ indicator could be entrained during sinoatrial cardiac activation in vivo. eCM grafts also increased conduction velocity and decreased the incidence of conduction block within the infarct. VT protection is critically dependent on expression of the gap-junction protein connexin 43 (Cx43; also known as Gja1): SMs genetically engineered to express Cx43 conferred a similar protection to that of eCMs against induced VT. Thus, engraftment of Cx43-expressing myocytes has the potential to reduce life-threatening post-infarct arrhythmias through the augmentation of intercellular coupling, suggesting autologous strategies for cardiac cell-based therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Laflamme, M. A. & Murry, C. E. Regenerating the heart. Nature Biotechnol. 23, 845–856 (2005)
Murry, C. E., Field, L. J. & Menasche, P. Cell-based cardiac repair: reflections at the 10-year point. Circulation 112, 3174–3183 (2005)
Wollert, K. C. et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet 364, 141–148 (2004)
Janssens, S. et al. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet 367, 113–121 (2006)
Smits, P. C. et al. Catheter-based intramyocardial injection of autologous skeletal myoblasts as a primary treatment of ischemic heart failure: clinical experience with six-month follow-up. J. Am. Coll. Cardiol. 42, 2063–2069 (2003)
Murry, C. E. et al. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature 428, 664–668 (2004)
Nygren, J. M. et al. Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nature Med. 10, 494–501 (2004)
Reinecke, H., Poppa, V. & Murry, C. E. Skeletal muscle stem cells do not transdifferentiate into cardiomyocytes after cardiac grafting. J. Mol. Cell. Cardiol. 34, 241–249 (2002)
Leobon, B. et al. Myoblasts transplanted into rat infarcted myocardium are functionally isolated from their host. Proc. Natl Acad. Sci. USA 100, 7808–7811 (2003)
Hagege, A. A. et al. Skeletal myoblast transplantation in ischemic heart failure: long-term follow-up of the first phase I cohort of patients. Circulation 114, I-108–I-113 (2006)
Solomon, S. D. et al. Sudden death in patients with myocardial infarction and left ventricular dysfunction, heart failure, or both. N. Engl. J. Med. 352, 2581–2588 (2005)
Henkel, D. M. et al. Ventricular arrhythmias after acute myocardial infarction: a 20-year community study. Am. Heart J. 151, 806–812 (2006)
Makkar, R. R., Lill, M. & Chen, P. S. Stem cell therapy for myocardial repair: is it arrhythmogenic? J. Am. Coll. Cardiol. 42, 2070–2072 (2003)
Soonpaa, M. H., Koh, G. Y., Klug, M. G. & Field, L. J. Formation of nascent intercalated disks between grafted fetal cardiomyocytes and host myocardium. Science 264, 98–101 (1994)
Roell, W. et al. Cellular cardiomyoplasty improves survival after myocardial injury. Circulation 105, 2435–2441 (2002)
Fleischmann, M. et al. Cardiac specific expression of the green fluorescent protein during early murine embryonic development. FEBS Lett. 440, 370–376 (1998)
Pouzet, B. et al. Is skeletal myoblast transplantation clinically relevant in the era of angiotensin-converting enzyme inhibitors? Circulation 104, I-223–I-228 (2001)
Dowell, J. D., Rubart, M., Pasumarthi, K. B., Soonpaa, M. H. & Field, L. J. Myocyte and myogenic stem cell transplantation in the heart. Cardiovasc. Res. 58, 336–350 (2003)
Tallini, Y. N. et al. Imaging cellular signals in the heart in vivo: Cardiac expression of the high-signal Ca2+ indicator GCaMP2. Proc. Natl Acad. Sci. USA 103, 4753–4758 (2006)
Pertsov, A. M., Davidenko, J. M., Salomonsz, R., Baxter, W. T. & Jalife, J. Spiral waves of excitation underlie reentrant activity in isolated cardiac muscle. Circ. Res. 72, 631–650 (1993)
Takahashi, T. et al. Optical mapping of the functional reentrant circuit of ventricular tachycardia in acute myocardial infarction. Heart Rhythm 1, 451–459 (2004)
Grant, A. O., Hondeghem, L. M. & Katzung, B. G. Effects of droperidol on depolarization-induced automaticity, maximum upstroke velocity (v max) and the kinetics of recovery of v max in guinea-pig ventricular myocardium. J. Pharmacol. Exp. Ther. 205, 193–203 (1978)
Baker, L. C., London, B., Choi, B. R., Koren, G. & Salama, G. Enhanced dispersion of repolarization and refractoriness in transgenic mouse hearts promotes reentrant ventricular tachycardia. Circ. Res. 86, 396–407 (2000)
Allessie, M. A. et al. Experimental electrophysiology and arrhythmogenicity. Anisotropy and ventricular tachycardia. Eur. Heart J. 10 (Suppl. E). E2–E8 (1989)
Salama, G., Kanai, A. & Efimov, I. R. Subthreshold stimulation of Purkinje fibers interrupts ventricular tachycardia in intact hearts. Experimental study with voltage-sensitive dyes and imaging techniques. Circ. Res. 74, 604–619 (1994)
Gaudesius, G., Miragoli, M., Thomas, S. P. & Rohr, S. Coupling of cardiac electrical activity over extended distances by fibroblasts of cardiac origin. Circ. Res. 93, 421–428 (2003)
Gnecchi, M. et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nature Med. 11, 367–368 (2005)
Kolossov, E. et al. Engraftment of engineered ES cell-derived cardiomyocytes but not BM cells restores contractile function to the infarcted myocardium. J. Exp. Med. 203, 2315–2327 (2006)
Roell, W. et al. Cellular cardiomyoplasty in a transgenic mouse model. Transplantation 73, 462–465 (2002)
Kreuzberg, M. M. et al. Connexin30.2 containing gap junction channels decelerate impulse propagation through the atrioventricular node. Proc. Natl Acad. Sci. USA 103, 5959–5964 (2006)
Pfeifer, A., Ikawa, M., Dayn, Y. & Verma, I. M. Transgenesis by lentiviral vectors: lack of gene silencing in mammalian embryonic stem cells and preimplantation embryos. Proc. Natl Acad. Sci. USA 99, 2140–2145 (2002)
Rubart, M. et al. Physiological coupling of donor and host cardiomyocytes after cellular transplantation. Circ. Res. 92, 1217–1224 (2003)
Miragoli, M., Gaudesius, G. & Rohr, S. Electrotonic modulation of cardiac impulse conduction by myofibroblasts. Circ. Res. 98, 801–810 (2006)
Berul, C. I., Aronovitz, M. J., Wang, P. J. & Mendelsohn, M. E. In vivo cardiac electrophysiology studies in the mouse. Circulation 94, 2641–2648 (1996)
Mitchell, G. F., Jeron, A. & Koren, G. Measurement of heart rate and Q-T interval in the conscious mouse. Basic Res. Cardiol. 274, H747–H751 (1998)
Schrickel, J. W. et al. Induction of atrial fibrillation in mice by rapid transesophageal atrial pacing. Basic Res. Cardiol. 97, 452–460 (2002)
Thevenaz, P., Ruttimann, U. E. & Unser, M. A pyramid approach to subpixel registration based on intensity. IEEE Trans. Image Process. 7, 27–41 (1998)
Choi, B. R. & Salama, G. Simultaneous maps of optical action potentials and calcium transients in guinea-pig hearts: mechanisms underlying concordant alternans. J. Physiol. (Lond.) 529, 171–188 (2000)
Gottlieb, G. J., Kubo, S. H. & Alonso, D. R. Ultrastructural characterization of the border zone surrounding early experimental myocardial infarcts in dogs. Am. J. Pathol. 103, 292–303 (1981)
Traub, O. et al. Immunochemical and electrophysiological characterization of murine connexin40 and -43 in mouse tissues and transfected human cells. Eur. J. Cell Biol. 64, 101–112 (1994)
Acknowledgements
We thank D. Fuerst for providing the anti-nebulin antibody; L. Field for providing the transgenic α-MHC–EGFP mouse line; W. Bloch for advice on immunostaining; C. Schaffer and N. Nishimura for technical advice; R. Gilmour Jr and N. Otani for comments on the manuscript; C. Fuegemann for the preparation of cardiac myofibroblasts; and H. Begerau, M. Czechowski, B. Eixmann, F. Holst, H. Doerr, K. Granitza and C. Russell for technical help. This study was supported by grants from the Deutsche Forschungsgemeinschaft (to W.R. and B.K.F.), the Federal Ministry of Education and Research, Germany (to T.L.), the European Commission (to B.K.F.), BONFOR (to J.W.S., A.H.) and the National Institutes of Health (to Y.N.T., M.I.K. and G.S.).
Author Contributions W.R., T.L. and P.S. contributed equally to this work. W.R. performed microsurgery, left-ventricular catheterization and analysis of data. T.L. supervised and analysed in vivo electrophysiology. P.S. performed in vivo imaging experiments, analysis and immunohistochemistry. Y.N.T. was involved in the in vivo imaging experiments and immunohistochemistry. B.-R.C. was involved in optical imaging and analysis of data. M.B. was involved in morphometry, immunohistochemistry and establishment of cardiac fibroblasts. R.D. was involved in the analysis of in vivo imaging experiments. U.B. was involved in the preparation of skeletal myoblasts, and in morphometry and immunohistochemistry. S.-M.H. was involved in the optical-imaging experiments and analysis of data. T.B. was involved in microsurgery, morphometry, left ventricular catheterization and immunohistochemistry. J.V.M. was involved in mouse breeding, immunohistochemistry and western blotting of tissues from the Cx43-expressing mouse. A.H. was involved in the generation of the lentivirus constructs. S.R. was involved in mouse breeding and in vivo imaging experiments. B.D. was involved in generation of the Cx43-expressing mouse model. B.G. was involved in Langendorff perfusion and optical-imaging experiments. A.P. supervised lentiviral work. A.W. supervised the microsurgery. G.S. was involved in the optical-imaging experiments, their analysis and writing of the manuscript. J.W.S. performed electrophysiological experiments in vivo and analysed data. M.I.K. designed experiments, analysed in vivo imaging experiments and wrote the manuscript. B.K.F. initiated the study, designed experiments and wrote the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-4 with Legends, Supplementary Table 1 and Supplementary Methods with references (PDF 5367 kb)
Supplementary Video 1
This file contains Supplementary Video 1 which shows in vivo monitoring of functional engraftment of GCaMP2+ embryonic cardiomyocytes in the infarct. Ca2+ signaling is detected within a section of infarcted left ventricle during sinus rhythm (heart rate: 490 bpm) in an anesthetized and ventilated mouse with open chest. Engrafted cells are coupled (2:1) to the native myocardium. GCaMP2 fluorescence only increases in case of coupling, the paradox movement of the infarcted area does not alter the fluorescence intensity. Recorded at 112 fps and displayed at 11.2 fps. (MOV 2570 kb)
Supplementary Video 2
This file contains Supplementary Video 2 which shows optical voltage recordings in infarcted, sham injected mouse. Di-4-ANEPPS fluorescence signals during S1-S2 stimulation protocol in an isolated heart. The first 2 waves are produced by stimulations at 150 ms, followed by early activation at 80 ms cycle length (white dot indicates S2 start). Note the movement of the voltage wave around the infarct area and the induction of prominent circular waves by S2 stimulation (see also Fig. 4d). Recorded at 1 kHz and displayed at 66 fps. (MOV 3690 kb)
Supplementary Video 3
This file contains Supplementary Video 3 which shows that engraftment of embryonic cardiomyocytes results in conduction of voltage wave into the infarct. Same stimulation protocol as in Supplementary Video 2. Note partial invasion of infarct by voltage wave during S2 stimulations and failure of S2 protocol (white dot) to produce boundary effects. Engraftment was confirmed by visualization of fluorescence of engrafted cells. Recorded at 1 kHz and displayed at 66 fps. (MOV 3448 kb)
Rights and permissions
About this article
Cite this article
Roell, W., Lewalter, T., Sasse, P. et al. Engraftment of connexin 43-expressing cells prevents post-infarct arrhythmia. Nature 450, 819–824 (2007). https://doi.org/10.1038/nature06321
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature06321
This article is cited by
-
COVID-19 HEART unveiling as atrial fibrillation: pathophysiology, management and future directions for research
The Egyptian Heart Journal (2023)
-
Structural and molecular changes in the rat myocardium following perfluoroctane sulfonate (PFOS) exposure are mitigated by quercetin via modulating HSP 70 and SERCA 2
Journal of Molecular Histology (2023)
-
Electrophysiological engineering of heart-derived cells with calcium-dependent potassium channels improves cell therapy efficacy for cardioprotection
Nature Communications (2021)
-
OptoGap is an optogenetics-enabled assay for quantification of cell–cell coupling in multicellular cardiac tissue
Scientific Reports (2021)
-
Cardiac optogenetics: a decade of enlightenment
Nature Reviews Cardiology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.