Abstract
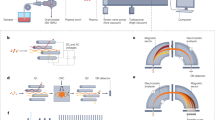
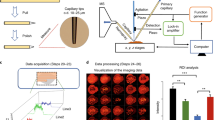
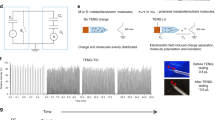
The ability of mass spectrometry to generate intact biomolecular ions efficiently in the gas phase has led to its widespread application in metabolomics1, proteomics2, biological imaging3, biomarker discovery4 and clinical assays (namely neonatal screens5). Matrix-assisted laser desorption/ionization6,7 (MALDI) and electrospray ionization8 have been at the forefront of these developments. However, matrix application complicates the use of MALDI for cellular, tissue, biofluid and microarray analysis and can limit the spatial resolution because of the matrix crystal size9 (typically more than 10 μm), sensitivity and detection of small compounds (less than 500 Da). Secondary-ion mass spectrometry10 has extremely high lateral resolution (100 nm) and has found biological applications11,12 although the energetic desorption/ionization is a limitation owing to molecular fragmentation. Here we introduce nanostructure-initiator mass spectrometry (NIMS), a tool for spatially defined mass analysis. NIMS uses ‘initiator’ molecules trapped in nanostructured surfaces or ‘clathrates’ to release and ionize intact molecules adsorbed on the surface. This surface responds to both ion and laser irradiation. The lateral resolution (ion-NIMS about 150 nm), sensitivity, matrix-free and reduced fragmentation of NIMS allows direct characterization of peptide microarrays, direct mass analysis of single cells, tissue imaging, and direct characterization of blood and urine.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Want, E. J., Cravatt, B. F. & Siuzdak, G. The expanding role of mass spectrometry in metabolite profiling and characterization. ChemBioChem 6, 1941–1951 (2005)
Aebersold, R. & Mann, M. Mass spectrometry-based proteomics. Nature 422, 198–207 (2003)
Chaurand, P., Stoeckli, M. & Caprioli, R. M. Direct profiling of proteins in biological tissue sections by MALDI mass spectrometry. Anal. Chem. 71, 5263–5270 (1999)
Lindon, J. C., Holmes, E. & Nicholson, J. K. Metabonomics in pharmaceutical R&D. FEBS J. 274, 1140–1151 (2007)
Chace, D. H. et al. Rapid diagnosis of phenylketonuria by quantitative analysis for phenylalanine and tyrosine in neonatal blood spots by tandem mass spectrometry. Clin. Chem. 39, 66–71 (1993)
Karas, M. & Hillenkamp, F. Laser desorption ionization of proteins with molecular masses exceeding 10,000 daltons. Anal. Chem. 60, 2299–2301 (1988)
Tanaka, K. et al. Protein and polymer analyses up to m/z 100 000 by laser ionization time-of flight mass spectrometry. Rapid Commun. Mass Spectrom. 2, 151–153 (1988)
Fenn, J. B., Mann, M., Meng, C. K., Wong, S. F. & Whitehouse, C. M. Electrospray ionization for mass spectrometry of large biomolecules. Science 246, 64–71 (1989)
Caldwell, R. L. & Caprioli, R. M. Tissue profiling by mass spectrometry: a review of methodology and applications. Mol. Cell. Proteomics 4, 394–401 (2005)
Benninghoven, A. in Secondary Ion Mass Spectrometry (eds Benninghoven, A., Rüdenauer, F. G. & Werner, H. W.) 1–6, 664–761 (Wiley, New York, 1987)
Kraft, M. L., Weber, P. K., Longo, M. L., Hutcheon, I. D. & Boxer, S. G. Phase separation of lipid membranes analyzed with high-resolution secondary ion mass spectrometry. Science 313, 1948–1951 (2006)
Ostrowski, S. G. et al. Secondary ion MS imaging of lipids in picoliter vials with a buckminsterfullerene ion source. Anal. Chem. 77, 6190–6196 (2005)
McLean, J. A., Stumpo, K. A. & Russell, D. H. Size-selected (2–10 nm) gold nanoparticles for matrix assisted laser desorption ionization of peptides. J. Am. Chem. Soc. 127, 5304–5305 (2005)
Wei, J., Buriak, J. M. & Siuzdak, G. Desorption–ionization mass spectrometry on porous silicon. Nature 399, 243–246 (1999)
Winograd, N. The magic of cluster SIMS. Anal. Chem. 77, 142A–149A (2005)
Wu, K. J. & Odom, R. W. Matrix-enhanced secondary ion mass spectrometry: A method for molecular analysis of solid surfaces. Anal. Chem. 68, 873–882 (1996)
Grade, H. & Cooks, R. G. Secondary ion mass-spectrometry—cationization of organic-molecules with metals. J. Am. Chem. Soc. 100, 5615–5621 (1978)
Overberg, A., Karas, M., Bahr, U., Kaufmann, R. & Hillenkamp, F. Matrix-assisted infrared-laser (2.94-μm) desorption ionization mass-spectrometry of large biomolecules. Rapid Commun. Mass Spectrom. 4, 293–296 (1990)
Singh-Gasson, S. et al. Maskless fabrication of light-directed oligonucleotide microarrays using a digital micromirror array. Nature Biotechnol. 17, 974–978 (1999)
Hashimoto, H., Nakamura, K., Takase, H., Okamoto, T. & Yamamoto, N. Quantitative TOF-SIMS imaging of DNA microarrays produced by bubble jet printing technique and the role of TOF-SIMS in life science industry. Appl. Surf. Sci. 231–2, 385–391 (2004)
Xu, J. Y., Braun, R. M. & Winograd, N. Applicability of imaging time-of flight secondary ion MS to the characterization of solid-phase synthesized combinatorial libraries. Anal. Chem. 75, 6155–6162 (2003)
Wiseman, J. M., Ifa, D. R., Song, Q. & Cooks, R. G. Tissue imaging at atmospheric pressure using desorption electrospray ionization (DESI) mass spectrometry. Angew. Chem. Int. Edn Engl. 45, 7188–7192 (2006)
Chaurand, P., Norris, J. L., Cornett, D. S., Mobley, J. A. & Caprioli, R. M. New developments in profiling and imaging of proteins from tissue sections by MALDI mass spectrometry. J. Proteome Res. 5, 2889–2900 (2006)
Hao, C., Clement, R. & Yang, P. Liquid chromatography–tandem mass spectrometry of bioactive pharmaceutical compounds in the aquatic environment—a decade’s activities. Anal. Bioanal. Chem. 387, 1247–1257 (2007)
Maurer, H. H. Position of chromatographic techniques in screening for detection of drugs or poisons in clinical and forensic toxicology and/or doping control. Clin. Chem. Lab. Med. 42, 1310–1324 (2004)
Fiedler, G. M. et al. Standardized peptidome profiling of human urine by magnetic bead separation and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Clin. Chem. 53, 421–428 (2007)
Villanueva, J. et al. Serum peptide profiling by magnetic particle-assisted, automated sample processing and MALDI-TOF mass spectrometry. Anal. Chem. 76, 1560–1570 (2004)
Kraemer, T. & Paul, L. D. Bioanalytical procedures for determination of drugs of abuse in blood. Anal. Bioanal. Chem. 388, 1415–1435 (2007)
Acknowledgements
We thank K. J. Wu and L. Wu for initial TOF–SIMS analysis; N. Winograd, A. Brock, B. Bothner, P. Kuhn and B. F. Cravatt for comments; J. Hoffmann and S. Head for peptide array preparation; the Kuhn laboratory for cell culture and imaging; B. Bowen for software development; D. Herr and J. Chun for tissue sections; the K. L. Turner laboratory for SEM imaging; and S. A. Trauger for nanospray ESI analysis. A.N. was supported by a postdoctoral fellowship from the Swedish Research Council (VR). We gratefully acknowledge financial support from the Department of Energy, the National Science Foundation, the National Cancer Institute and the National Institutes of Health.
Author Contributions T.R.N. and O.Y. contributed equally to this work. T.R.N. and G.S. conceived of NIMS, developed and applied NIMS, designed experiments, analysed data, and wrote the manuscript. O.Y. developed and applied NIMS, designed experiments, analysed data, and wrote the manuscript. M.T.N. performed SEM studies. D.M. prepared cell cultures and performed fluorescent imaging. A.N. and W.U. developed and applied NIMS. J.A. used NIMS to image mouse embryo. S.L.G. performed ion-NIMS.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
The file contains Supplementary Discussion, Supplementary Figures 1-11 with Legends, Supplementary Tables 1-2, Supplementary Methods (including illustrated step-by-step procedures) and Legends for Supplementary Videos 1-8. The Supplementary Discussion includes: in depth discussion of the single cell results by laser-NIMS and its comparison with MALDI, nanoESI and ion-NIMS. Supplementary Figure 1 is a schematic to the main findings and applications of NIMS to aid readers unfamiliar with the immediate discipline. The Supplementary Figures 2-11 and the 2 Supplementary Tables provide results to proof and better understand assertions made throughout the printed version of the paper. Supplementary Methods contains a detailed step-by-step protocol to make NIMS chips. Some images are provided, as well as 7 Supplementary Videos (2-8) to visualize and facilitate the performance of this protocol. Supplementary Video 1 concerns NIMS mechanism and shows the reversible migration of the initiator within the nanostructure surface. (PDF 1264 kb)
Supplementary Movie 1
The file contains Supplementary Movie 1 which shows perfluorinated initiator reversibly migrating out of the nanostructured surface in response to heating. (MOV 5241 kb)
Supplementary Movie 2
The file contains Supplementary Movie 2 which shows silicon wafer cutting with a diamond-tip scribe. (MOV 1885 kb)
Supplementary Movie 3
The file contains Supplementary Movie 3 which shows washing the wafer chip with methanol and blowing it off with UHP nitrogen. (MOV 1280 kb)
Supplementary Movie 4
The file contains Supplementary Movie 4 which shows assembling the teflon cell with the silicon wafer chip. (MOV 3096 kb)
Supplementary Movie 5
The file contains Supplementary Movie 5 which shows post-etching: teflon cell disassembling and washing. (MOV 3438 kb)
Supplementary Movie 6
The file contains Supplementary Movie 6 which shows washing and drying the chip with UHP nitrogen. (MOV 1834 kb)
Supplementary Movie 7
The file contains Supplementary Movie 7 which shows adding the initiator compound to the etched chip. (MOV 2606 kb)
Supplementary Movie 8
The file contains Supplementary Movie 8 which shows removing the excess of initiator compound with UHP nitrogen. (MOV 4132 kb)
Rights and permissions
About this article
Cite this article
Northen, T., Yanes, O., Northen, M. et al. Clathrate nanostructures for mass spectrometry. Nature 449, 1033–1036 (2007). https://doi.org/10.1038/nature06195
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature06195
This article is cited by
-
Subcellular quantitative imaging of metabolites at the organelle level
Nature Metabolism (2023)
-
Plasmonic polydopamine-modified TiO2 nanotube substrates for surface-assisted laser desorption/ionization mass spectrometry imaging
Nano Research (2023)
-
Super-assembled sandwich-like Au@MSN@Ag nanomatrices for high-throughput and efficient detection of small biomolecules
Nano Research (2022)
-
A multiplexed nanostructure-initiator mass spectrometry (NIMS) assay for simultaneously detecting glycosyl hydrolase and lignin modifying enzyme activities
Scientific Reports (2021)
-
Function-driven single-cell genomics uncovers cellulose-degrading bacteria from the rare biosphere
The ISME Journal (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.