Abstract
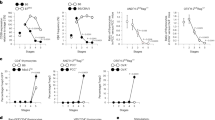
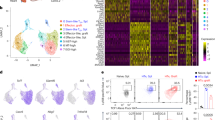
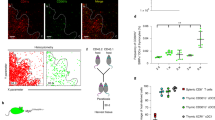
In cellular immunology the critical balance between effector and regulatory mechanisms is highlighted by serious immunopathologies attributable to mutations in Foxp3, a transcription factor required for a major subset of regulatory T (Tr) cells1,2,3. Thus, many studies have focused on the developmental origin of Tr cells, with the prevailing view that they emerge in the thymus from late-stage T-cell progenitors whose T-cell receptors (TCRs) engage high affinity (agonist) ligands4,5,6. This study questions the completeness of that interpretation. Here we show that without any obvious effect on TCR-mediated selection, the normal differentiation of mouse γδ T cells into potent cytolytic and interferon-γ-secreting effector cells is switched towards an aggregate regulatory phenotype by limiting the capacity of CD4+CD8+ T-cell progenitors to influence in trans early γδ cell progenitors. Unexpectedly, we found that the propensity of early TCR-αβ+ progenitors to differentiate into Foxp3+ Tr cells is also regulated in trans by CD4+CD8+ T-cell progenitor cells, before agonist selection.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hori, S., Nomura, T. & Sakaguchi, S. Control of regulatory T cell development by the transcription factor Foxp3. Science 299, 1057–1061 (2003)
Fontenot, J. D., Gavin, M. A. & Rudensky, A. Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nature Immunol. 4, 330–336 (2003)
Khattri, R., Cox, T., Yasayko, S. A. & Ramsdell, F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nature Immunol. 4, 337–342 (2003)
Jordan, M. S. et al. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nature Immunol. 2, 301–306 (2001)
Apostolou, I., Sarukhan, A., Klein, L. & von Boehmer, H. Origin of regulatory T cells with known specificity for antigen. Nature Immunol. 3, 756–763 (2002)
Fontenot, J. D. et al. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity 22, 329–341 (2005)
Silva-Santos, B., Pennington, D. J. & Hayday, A. C. Lymphotoxin-mediated regulation of γδ cell differentiation by αβ T cell progenitors. Science 307, 925–928 (2005)
Havran, W. L. & Allison, J. P. Origin of Thy-1+ dendritic epidermal cells of adult mice from fetal thymic precursors. Nature 344, 68–70 (1990)
Girardi, M. et al. Resident skin-specific γδ T cells provide local, nonredundant regulation of cutaneous inflammation. J. Exp. Med. 195, 855–867 (2002)
Wise, E., Lewis, J., LeClercq, G., Tigelaar, R. E. & Hayday, A. C. Molecular analysis of skin-resident T cells clarifies functional potential, heterogeneity, strain variation and major differences to gut intraepithelial T cells. J. Immunol. (submitted).
Suri-Payer, E., Amar, A. Z., Thornton, A. M. & Shevach, E. M. CD4+CD25+ T cells inhibit both the induction and effector function of autoreactive T cells and represent a unique lineage of immunoregulatory cells. J. Immunol. 160, 1212–1218 (1998)
Sakaguchi, S., Sakaguchi, N., Asano, M., Itoh, M. & Toda, M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor α-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 155, 1151–1164 (1995)
Livak, F., Wilson, A., MacDonald, H. R. & Schatz, D. G. αβ lineage-committed thymocytes can be rescued by the γδ T cell receptor (TCR) in the absence of TCRβ chain. Eur. J. Immunol. 27, 2948–2958 (1997)
Passoni, L. et al. Intrathymic δ selection events in γδ cell development. Immunity 7, 83–95 (1997)
Buer, J., Aifantis, I., DiSanto, J. P., Fehling, H. J. & von Boehmer, H. Role of different T cell receptors in the development of pre-T cells. J. Exp. Med. 185, 1541–1547 (1997)
Fehling, H. J., Krotkova, A., Saint-Ruf, C. & von Boehmer, H. Crucial role of the pre-T-cell receptor α gene in development of αβ but not γδ T cells. Nature 375, 795–798 (1995)
Gibbons, D. et al. The biological activity of natural and mutant pTα alleles. J. Exp. Med. 194, 695–703 (2001)
Prockop, S. E. & Petrie, H. T. Regulation of thymus size by competition for stromal niches among early T cell progenitors. J. Immunol. 173, 1604–1611 (2004)
Fontenot, J. D., Rasmussen, J. P., Gavin, M. A. & Rudensky, A. Y. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nature Immunol. 6, 1142–1151 (2005)
Zelenika, D. et al. Regulatory T cells overexpress a subset of Th2 gene transcripts. J. Immunol. 168, 1069–1079 (2002)
Read, S., Malmstrom, V. & Powrie, F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25+CD4+ regulatory cells that control intestinal inflammation. J. Exp. Med. 192, 295–302 (2000)
Takahashi, T. et al. Immunologic self-tolerance maintained by CD25+CD4+ regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J. Exp. Med. 192, 303–310 (2000)
Carter, J. D., Calabrese, G. M., Naganuma, M. & Lorenz, U. Deficiency of the Src homology region 2 domain-containing phosphatase 1 (SHP-1) causes enrichment of CD4+CD25+ regulatory T cells. J. Immunol. 174, 6627–6638 (2005)
van Santen, H. M., Benoist, C. & Mathis, D. Number of T reg cells that differentiate does not increase upon encounter of agonist ligand on thymic epithelial cells. J. Exp. Med. 200, 1221–1230 (2004)
Lerman, M. A., Larkin, J., Cozzo, C., Jordan, M. S. & Caton, A. J. CD4+ CD25+ regulatory T cell repertoire formation in response to varying expression of a neo-self-antigen. J. Immunol. 173, 236–244 (2004)
Aluvihare, V. R., Kallikourdis, M. & Betz, A. G. Regulatory T cells mediate maternal tolerance to the fetus. Nature Immunol. 5, 266–271 (2004)
Futterer, A., Mink, K., Luz, A., Kosco-Vilbois, M. H. & Pfeffer, K. The lymphotoxin β receptor controls organogenesis and affinity maturation in peripheral lymphoid tissues. Immunity 9, 59–70 (1998)
Philpott, K. L. et al. Lymphoid development in mice congenitally lacking T cell receptor αβ-expressing cells. Science 256, 1448–1452 (1992)
Mombaerts, P. et al. Mutations in T-cell antigen receptor genes α and β block thymocyte development at different stages. Nature 360, 225–231 (1992)
Bosco, N., Agenes, F., Rolink, A. G. & Ceredig, R. Peripheral T cell lymphopenia and concomitant enrichment in naturally arising regulatory T cells: The case of the pre-Tα gene-deleted mouse. J. Immunol. 177, 5014–5023 (2006)
Acknowledgements
The authors thank the Wellcome Trust for support, and W. Turnbull, E. Wise, G. Warnes, T. Tree, L. Graca, A. Aguirre, P. Pereira, D. Gibbons, S. Haque, Jian-Guo Chai, H. Brady, C. Langford, P. Ellis, J. Lewis, M. Girardi and R. Tigelaar for assistance, support and discussions. T.S. is supported by a Ph.D. scholarship from the Boehringer Ingelheim Fonds.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests.
Supplementary information
Supplementary Figure 1
FACS profiles demonstrating that splenic yδ cells from pTα-deficient mice express similar Vy chains to those from WT animals.
Supplementary Figure 2
FACS profiles demonstrating that yδ cells from TCRβ-/- and pTα-/- mice express Foxp3.
Supplementary Figure 3
Data demonstrating that ICER expression can subset DN2 thymocytes.
Supplementary Figure 4
Representation of the data obtained from transcriptional profiling of DN2 cells from WT (DN2-L and DN2-S) and pTα-/- mice demonstrating that WT DN2-S cells are more similar to pTα-/- DN2 cells than to WT DN2-L cells.
Supplementary Figure 5
A revised scheme for regulatory T cell development.
Supplementary Table 1
Table of absolute cell number of cell populations from the thymus, spleen, or small intestine (Gut) of wild type or pTα-/- mice.
Supplementary Table 2
Table showing the percentage of TCRαβ+CD4+CD8α- [CD4+ SP] thymocytes from age-matched wild type mice [WT] (n=3) and pTα-/- mice (n=5) that express TCR chains that include either Vα2, Vβ3, Vβ5.1/5.2 or Vβ8.1/8.2.
Supplementary Table 3
Table illustrating a selection of genes overexpressed in WT DN2 cells compared to pTα-/- DN2 cells
Supplementary Table 4
Primers used for semi-quantitative and Real-Time PCR.
Supplementary Figure Legends
This file contains text to accompany the above Supplementary Figures and Supplementary Tables
Rights and permissions
About this article
Cite this article
Pennington, D., Silva-Santos, B., Silberzahn, T. et al. Early events in the thymus affect the balance of effector and regulatory T cells. Nature 444, 1073–1077 (2006). https://doi.org/10.1038/nature06051
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature06051
This article is cited by
-
Suppressive activity of Vδ2+ γδ T cells on αβ T cells is licensed by TCR signaling and correlates with signal strength
Cancer Immunology, Immunotherapy (2020)
-
Expanding Diversity and Common Goal of Regulatory T and B Cells. I: Origin, Phenotype, Mechanisms
Archivum Immunologiae et Therapiae Experimentalis (2017)
-
IL-2 and IL-15 dependent thymic development of Foxp3-expressing regulatory T lymphocytes
Protein & Cell (2017)
-
Myocyte-derived Tnfsf14 is a survival factor necessary for myoblast differentiation and skeletal muscle regeneration
Cell Death & Disease (2015)
-
Associated Immunological Disorders and Cellular Immune Dysfunction in Thymoma: A Study of 87 Cases from Thailand
Archivum Immunologiae et Therapiae Experimentalis (2013)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.