Abstract
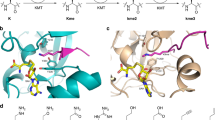
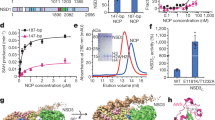
Post-translational histone modification has a fundamental role in chromatin biology and is proposed to constitute a ‘histone code’ in epigenetic regulation1,2. Differential methylation of histone H3 and H4 lysyl residues regulates processes including heterochromatin formation, X-chromosome inactivation, genome imprinting, DNA repair and transcriptional regulation3. The discovery of lysyl demethylases using flavin (amine oxidases)4 or Fe(ii) and 2-oxoglutarate as cofactors (2OG oxygenases)5,6,7 has changed the view of methylation as a stable epigenetic marker. However, little is known about how the demethylases are selective for particular lysyl-containing sequences in specific methylation states, a key to understanding their functions. Here we reveal how human JMJD2A (jumonji domain containing 2A), which is selective towards tri- and dimethylated histone H3 lysyl residues 9 and 36 (H3K9me3/me2 and H3K36me3/me2), discriminates between methylation states and achieves sequence selectivity for H3K9. We report structures of JMJD2A–Ni(ii)–Zn(ii) inhibitor complexes bound to tri-, di- and monomethyl forms of H3K9 and the trimethyl form of H3K36. The structures reveal a lysyl-binding pocket in which substrates are bound in distinct bent conformations involving the Zn-binding site. We propose a mechanism for achieving methylation state selectivity involving the orientation of the substrate methyl groups towards a ferryl intermediate. The results suggest distinct recognition mechanisms in different demethylase subfamilies and provide a starting point to develop chemical tools for drug discovery and to study and dissect the complexity of reversible histone methylation and its role in chromatin biology.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Margueron, R., Trojer, P. & Reinberg, D. The key to development: interpreting the histone code? Curr. Opin. Genet. Dev. 15, 163–176 (2005)
Zhang, Y. & Reinberg, D. Transcription regulation by histone methylation: interplay between different covalent modifications of the core histone tails. Genes Dev. 15, 2343–2360 (2001)
Martin, C. & Zhang, Y. The diverse functions of histone lysine methylation. Nature Rev. Mol. Cell Biol. 6, 838–849 (2005)
Shi, Y. et al. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell 119, 941–953 (2004)
Tsukada, Y. et al. Histone demethylation by a family of JmjC domain-containing proteins. Nature 439, 811–816 (2006)
Klose, R. J., Kallin, E. M. & Zhang, Y. JmjC-domain-containing proteins and histone demethylation. Nature Rev. Genet. 7, 715–727 (2006)
Whetstine, J. R. et al. Reversal of histone lysine trimethylation by the JMJD2 family of histone demethylases. Cell 125, 467–481 (2006)
Flashman, E. & Schofield, C. J. The most versatile of all reactive intermediates? Nature Chem. Biol. 3, 86–87 (2007)
Chen, Z. et al. Structural insights into histone demethylation by JMJD2 family members. Cell 125, 691–702 (2006)
Clifton, I. J. et al. Structural studies on 2-oxoglutarate oxygenases and related double-stranded β-helix fold proteins. J. Inorg. Biochem. 100, 644–669 (2006)
Lo, W. S. et al. Snf1–a histone kinase that works in concert with the histone acetyltransferase Gcn5 to regulate transcription. Science 293, 1142–1146 (2001)
Nelson, C. J., Santos-Rosa, H. & Kouzarides, T. Proline isomerization of histone H3 regulates lysine methylation and gene expression. Cell 126, 905–916 (2006)
Price, J. C., Barr, E. W., Tirupati, B., Bollinger, J. M. & Krebs, C. Kinetic dissection of the catalytic mechanism of taurine:α-ketoglutarate dioxygenase (TauD) from Escherichia coli. Biochemistry 42, 7497–7508 (2003)
Grzyska, P. K. et al. Steady-state and transient kinetic analyses of taurine/α-ketoglutarate dioxygenase: effects of oxygen concentration, alternative sulfonates, and active-site variants on the FeIV-oxo intermediate. Biochemistry 44, 3845–3855 (2005)
Elkins, J. M. et al. Structure of Factor-inhibiting Hypoxia-inducible Factor (HIF) reveals mechanism of oxidative modification of HIF-1. J. Biol. Chem. 278, 1802–1806 (2003)
Liang, G., Klose, R. J., Gardner, K. E. & Zhang, Y. Yeast Jhd2p is a histone H3 Lys4 trimethyl demethylase. Nature Struct. Mol. Biol. 14, 243–245 (2007)
Seward, D. J. et al. Demethylation of trimethylated histone H3 Lys4 in vivo by JARID1 JmjC proteins. Nature Struct. Mol. Biol. 14, 240–242 (2007)
Acknowledgements
We thank A. Edwards and J. Min for critical discussions and reading of the manuscript; P. Siegbahn for support; and F. Sobott, K. Di Gleria and C. Webby for technical help with MALDI–TOF analysis. The Structural Genomics Consortium is a registered charity funded by the Wellcome Trust, GlaxoSmithKline, Genome Canada, the Canadian Institutes of Health Research, the Ontario Innovation Trust, the Ontario Research and Development Challenge Fund, the Canadian Foundation for Innovation, Vinnova, Knut and Alice Wallenberg foundation and Karolinska Institutet. C.J.S. and co-workers are funded by the Wellcome Trust, and the Biotechnology and Biological Sciences Research Council, United Kingdom. Atomic coordinates and structure factors for the reported crystal structures have been deposited with the Protein Data Bank under accession codes 2oq7 (JMJD2A–NOG–Ni(II)), 2oq6 (JMJD2A–NOG–Ni(II)–H3K9me3), 2os2(JMJD2A–NOG–Ni(II)–H3K36me3), 2ot7(JMJD2A–NOG–Ni(II)–H3K9me1) and 2ox0 (JMJD2A–NOG–Ni(II)–H3K9me2).
Author Contributions S.S.N. purified, crystallized, collected data and performed MALDI–TOF analyses; K.L.K., M.A.M. and E.S.P. collected, processed and refined X-ray data; D.B. and B.M.R.L. performed MS analysis; P.S. and O.G. cloned the construct; F.v.D. collected data; N.R.R., J.C.S. and J.O. synthesized peptides; T.B. performed MD studies; J.E.B. performed bioinformatic analyses; M.S. was involved in study design; C.J.S. and U.O. designed the study, analysed data and wrote the paper. M.A.M. and D.B. contributed equally to the study. All authors discussed the results and commented on the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
C.J.S. is a co-founder and director of ReOx, a company that aims to exploit knowledge about the hypoxic response for therapeutic benefit.
Supplementary information
Supplementary Information
This file contains Supplementary Tables S1-S2 and Supplementary Figures S1-S10 with Legends. (PDF 1841 kb)
Rights and permissions
About this article
Cite this article
Ng, S., Kavanagh, K., McDonough, M. et al. Crystal structures of histone demethylase JMJD2A reveal basis for substrate specificity. Nature 448, 87–91 (2007). https://doi.org/10.1038/nature05971
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature05971
This article is cited by
-
KDM4A, involved in the inflammatory and oxidative stress caused by traumatic brain injury-hemorrhagic shock, partly through the regulation of the microglia M1 polarization
BMC Neuroscience (2023)
-
In-silico guided chemical exploration of KDM4A fragments hits
Clinical Epigenetics (2023)
-
Reading and erasing of the phosphonium analogue of trimethyllysine by epigenetic proteins
Communications Chemistry (2022)
-
Structural analysis of the 2-oxoglutarate binding site of the circadian rhythm linked oxygenase JMJD5
Scientific Reports (2022)
-
Rebelled epigenome: histone H3S10 phosphorylation and H3S10 kinases in cancer biology and therapy
Clinical Epigenetics (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.