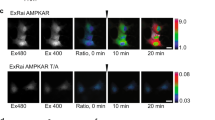
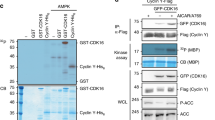
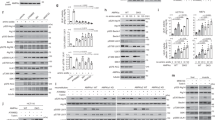
Abstract
AMP-activated protein kinase (AMPK, also known as SNF1A) has been primarily studied as a metabolic regulator that is activated in response to energy deprivation1. Although there is relatively ample information on the biochemical characteristics of AMPK, not enough data exist on the in vivo function of the kinase. Here, using the Drosophila model system, we generated the first animal model with no AMPK activity and discovered physiological functions of the kinase. Surprisingly, AMPK-null mutants were lethal with severe abnormalities in cell polarity and mitosis, similar to those of lkb1-null mutants. Constitutive activation of AMPK restored many of the phenotypes of lkb1-null mutants, suggesting that AMPK mediates the polarity- and mitosis-controlling functions of the LKB1 serine/threonine kinase. Interestingly, the regulatory site of non-muscle myosin regulatory light chain (MRLC; also known as MLC2)2,3 was directly phosphorylated by AMPK. Moreover, the phosphomimetic mutant of MRLC3 rescued the AMPK-null defects in cell polarity and mitosis, suggesting MRLC is a critical downstream target of AMPK. Furthermore, the activation of AMPK by energy deprivation was sufficient to cause dramatic changes in cell shape, inducing complete polarization and brush border formation in the human LS174T cell line, through the phosphorylation of MRLC. Taken together, our results demonstrate that AMPK has highly conserved roles across metazoan species not only in the control of metabolism, but also in the regulation of cellular structures.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kahn, B. B., Alquier, T., Carling, D. & Hardie, D. G. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metabol. 1, 15–25 (2005)
Matsumura, F. Regulation of myosin II during cytokinesis in higher eukaryotes. Trends Cell Biol. 15, 371–377 (2005)
Jordan, P. & Karess, R. E. Myosin light chain-activating phosphorylation sites are required for oogenesis in Drosophila. J. Cell Biol. 139, 1805–1819 (1997)
Pan, D. A. & Hardie, D. G. A homologue of AMP-activated protein kinase in Drosophila melanogaster is sensitive to AMP and is activated by ATP depletion. Biochem. J. 367, 179–186 (2002)
Knust, E. & Bossinger, O. Composition and formation of intercellular junctions in epithelial cells. Science 298, 1955–1959 (2002)
Hay, B. A., Wolff, T. & Rubin, G. M. Expression of baculovirus p35 prevents cell death in Drosophila. Development 120, 2121–2129 (1994)
Alessi, D. R., Sakamoto, K. & Bayascas, J. R. LKB1-dependent signaling pathways. Annu. Rev. Biochem. 75, 137–163 (2006)
Baas, A. F. et al. Complete polarization of single intestinal epithelial cells upon activation of LKB1 by STRAD. Cell 116, 457–466 (2004)
Martin, S. G. & St Johnston, D. A role for Drosophila LKB1 in anterior–posterior axis formation and epithelial polarity. Nature 421, 379–384 (2003)
Bettencourt-Dias, M. et al. Genome-wide survey of protein kinases required for cell cycle progression. Nature 432, 980–987 (2004)
Ivanov, A. I., Hunt, D., Utech, M., Nusrat, A. & Parkos, C. A. Differential roles for actin polymerization and a myosin II motor in assembly of the epithelial apical junctional complex. Mol. Biol. Cell 16, 2636–2650 (2005)
Edwards, K. A. & Kiehart, D. P. Drosophila nonmuscle myosin II has multiple essential roles in imaginal disc and egg chamber morphogenesis. Development 122, 1499–1511 (1996)
Michell, B. J. et al. Isoform-specific purification and substrate specificity of the 5′-AMP-activated protein kinase. J. Biol. Chem. 271, 28445–28450 (1996)
Karess, R. E. et al. The regulatory light chain of nonmuscle myosin is encoded by spaghetti-squash, a gene required for cytokinesis in Drosophila. Cell 65, 1177–1189 (1991)
Luo, Z., Saha, A. K., Xiang, X. & Ruderman, N. B. AMPK, the metabolic syndrome and cancer. Trends Pharmacol. Sci. 26, 69–76 (2005)
Lee, J. H. et al. In vivo p53 function is indispensable for DNA damage-induced apoptotic signaling in Drosophila. FEBS Lett. 550, 5–10 (2003)
Lee, J. H. et al. JNK pathway mediates apoptotic cell death induced by tumor suppressor LKB1 in Drosophila. Cell Death Differ. 13, 110–1122 (2006)
Lee, A. & Treisman, J. E. Excessive myosin activity in Mbs mutants causes photoreceptor movement out of the Drosophila eye disc epithelium. Mol. Biol. Cell 15, 3285–3295 (2004)
Sakurada, K. et al. Dynamics of myosin light chain phosphorylation at Ser19 and Thr18/Ser19 in smooth muscle cells in culture. Am. J. Physiol. 274, 1563–1572 (1998)
Park, J. et al. Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature 441, 1157–1161 (2006)
Monfar, M. et al. Activation of pp70/85 S6 kinases in interleukin-2-responsive lymphoid cells is mediated by phosphatidylinositol 3-kinase and inhibited by cyclic AMP. Mol. Cell. Biol. 15, 326–337 (1995)
Acknowledgements
We would like to thank A. Wodarz, K. L. Guan, R. Fehon, C. Sunkel, R. Saint, H. Piwnica-Worms, D. St Johnston, C. Q. Doe, R. T. Moon, M. Montminy, L. Alphey and H. Clever, as well as the Bloomington Stock Center, Developmental Studies Hybridoma Bank and Drosophila Genomics Research Center, for kindly providing materials. We also thank the Korea Basic Science Institute for electron microscopy analyses and the Korean Cell Line Bank for LS174T cell stock. This research was supported by a National Creative Research Initiatives grant from the Korean Ministry of Science and Technology/KOSEF.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
J.C. and J.K. have stocks in GenExel Incorporation.
Supplementary information
Supplementary Information
This file contains Supplementary Methods, Supplementary Figures 1-16 with Legends and additional references. (PDF 2787 kb)
Rights and permissions
About this article
Cite this article
Lee, J., Koh, H., Kim, M. et al. Energy-dependent regulation of cell structure by AMP-activated protein kinase. Nature 447, 1017–1020 (2007). https://doi.org/10.1038/nature05828
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature05828
This article is cited by
-
Modulation of colonic immunometabolic responses during Clostridioides difficile infection ameliorates disease severity and inflammation
Scientific Reports (2023)
-
Rapid metabolic reprogramming mediated by the AMP-activated protein kinase during the lytic cycle of Toxoplasma gondii
Nature Communications (2023)
-
Valproate-induced murine autism spectrum disorder is associated with dysfunction of amygdala parvalbumin interneurons and downregulation of AMPK/SIRT1/PGC1α signaling
Metabolic Brain Disease (2023)
-
The beta subunit of AMP-activated protein kinase is critical for cell cycle progression and parasite development in Toxoplasma gondii
Cellular and Molecular Life Sciences (2022)
-
Crosstalk between mechanotransduction and metabolism
Nature Reviews Molecular Cell Biology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.