Abstract
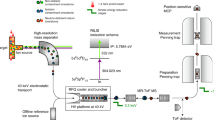
The heaviest elements to have been chemically characterized are seaborgium1 (element 106), bohrium2 (element 107) and hassium3 (element 108). All three behave according to their respective positions in groups 6, 7 and 8 of the periodic table, which arranges elements according to their outermost electrons and hence their chemical properties. However, the chemical characterization results are not trivial: relativistic effects on the electronic structure of the heaviest elements can strongly influence chemical properties4,5,6. The next heavy element targeted for chemical characterization is element 112; its closed-shell electronic structure with a filled outer s orbital suggests that it may be particularly susceptible to strong deviations from the chemical property trends expected within group 12. Indeed, first experiments concluded that element 112 does not behave like its lighter homologue mercury7,8,9. However, the production and identification methods10,11 used cast doubt on the validity of this result. Here we report a more reliable chemical characterization of element 112, involving the production of two atoms of 283112 through the alpha decay of the short-lived 287114 (which itself forms in the nuclear fusion reaction12 of 48Ca with 242Pu) and the adsorption of the two atoms on a gold surface. By directly comparing the adsorption characteristics of 283112 to that of mercury and the noble gas radon, we find that element 112 is very volatile and, unlike radon, reveals a metallic interaction with the gold surface. These adsorption characteristics establish element 112 as a typical element of group 12, and its successful production unambiguously establishes the approach to the island of stability of superheavy elements through 48Ca-induced nuclear fusion reactions with actinides.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Schädel, M. et al. Chemical properties of element 106 (seaborgium). Nature 388, 55–57 (1997)
Eichler, R. et al. Chemical characterization of bohrium (element 107). Nature 407, 63–65 (2000)
Düllmann et al. Chemical investigation of hassium (Hs, element 108). Nature 418, 859–862 (2002)
Fricke, B. Superheavy elements. Structure Bonding 21, 90–144 (1975)
Pyykkö, P. & Desclaux, J.-P. Relativity and the periodic system of elements. Acc. Chem. Res. 12, 276–281 (1979)
Schwerdtfeger, P. & Seth, M. in Encyclopaedia of Computational Chemistry Vol. 4 2480–2499 (Wiley, New York, 1998)
Yakushev, A. B. et al. First attempt to chemically identify element 112. Radiochim. Acta 89, 743–745 (2001)
Yakushev, A. B. et al. Chemical identification and properties of element 112. Radiochim. Acta 91, 433–440 (2003)
Gäggeler, H. W. et al. Chemical and nuclear studies of hassium and element 112. Nucl. Phys. A 734, 208–212 (2004)
Gregorich, K. E. et al. Attempt to confirm superheavy element production in the 48Ca + 238U reaction. Phys. Rev. C 72, 014605 (2005)
Eichler, R. et al. Attempts to chemically investigate element 112. Radiochim. Acta 94, 181–191 (2006)
Oganessian et al. Measurements of cross sections and decay properties of the isotopes of elements 112, 114, and 116 produced in the fusion reactions 233,238U, 242Pu, and 248Cm+48Ca. Phys. Rev. C 70, 064609 (2004)
Hofmann, S. et al. The new element 112. Z. Phys. A 354, 229–230 (1996)
Oganessian et al. Synthesis of nuclei of the superheavy element 114 in reactions induced by 48Ca. Nature 400, 242–245 (1999)
Oganessian et al. Second experiment at VASSILISSA separator on the synthesis of the element 112. Eur. Phys. J. A 19, 3–6 (2004)
Eichler, B. Volatility properties of transactinides around Z = 114 (prediction). Kernenergie [in German]. 19, 307–311 (1976)
Pitzer, K. S. Are elements 112, 114, and 118 relatively inert gases? J. Chem. Phys. 63, 1032–1333 (1975)
Gaston, N., Opahle, I., Gäggeler, H. W. & Schwerdtfeger, P. Is eka-mercury (element 112) a group 12 metal? Angew. Chem. Int. Edn 46 (14). 2444–2447 (2007)
Eichler, B. & Rossbach, H. Adsorption of volatile metals on metal surfaces and its application in nuclear chemistry. I. Calculation of adsorption enthalpies for hypothetical superheavy elements with Z around 114. Radiochim. Acta 33, 121–125 (1983)
Eliav, E., Kaldor, U. & Ishikawa, Y. Transition energies of mercury and ekamercury (element 112) by the relativistic coupled-cluster method. Phys. Rev. A 52, 2765–2769 (1995)
Seth, M., Schwerdtfeger, P. & Dolg, M. The chemistry of the superheavy elements. I Pseudopotentials for 111 and 112 and relativistic coupled clusters calculations for (112)H+, (112)F2, and (112)F4 . J. Chem. Phys. 106, 3623–3632 (1997)
Pershina, V., Bastug, T., Jacob, T., Fricke, B. & Varga, S. Intermetallic compounds of the heaviest elements: the electronic structure and bonding of dimers of element 112 and its homolog Hg. Chem. Phys. Lett. 365, 176–183 (2002)
Sarpe-Tudoran, C. et al. Adsorption of super-heavy elements on metal surfaces. Eur. Phys. J. D 24, 65–67 (2003)
Pershina, V. & Bastug, T. Relativistic effects on experimentally studied gas-phase properties of the heaviest elements. Chem. Phys. 311, 139–150 (2005)
Eichler, B. Metal Chemistry of Transactinides. PSI Report 00–09, ISSN 1019–0643 (Villigen, 2000)
Eichler, B. Volatilization Properties of Transactinides from Metal Surfaces and Melts (Thermochemical Calculation). PSI Report 03–01, ISSN 1019–0643 (Villigen, 2002)
Eichler, R. & Schädel, M. Adsorption of radon on metal surfaces: a model study for chemical investigations of elements 112 and 114. J. Phys. Chem. B 106, 5413–5420 (2002)
Düllmann et al. IVO, a device for in situ volatilization and on-line detection of products from heavy ion reactions. Nucl. Instrum. Meth. A 479, 631–639 (2002)
Soverna, S. et al. Thermochromatographic studies of mercury and radon on transition metal surfaces. Radiochim. Acta 93, 1–8 (2005)
Zvara, I. Simulation of thermochromatographic processes by the Monte Carlo method. Radiochim. Acta 38, 95–101 (1985)
Eichler, B., Zimmermann, P. & Gäggeler, H. W. Adsorption of radon on ice surfaces. J. Phys. Chem. A 104, 3126–3131 (2000)
Acknowledgements
We are indebted to the staff of the U-400 cyclotron for providing intense beams of 48Ca. This work was supported in part by the Russian Foundation for Basic Research and by the Swiss National Science Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Notes and Supplementary Figures 1-7 describing the Monte-Carlo approach for the determination of the adsorption enthalpy of single atom amounts together with the statistical procedure applied to evaluate the adsorption enthalpy of element 112 on gold. Further details regarding the background of the measurement and a random rate analysis are included. (PDF 530 kb)
Rights and permissions
About this article
Cite this article
Eichler, R., Aksenov, N., Belozerov, A. et al. Chemical characterization of element 112. Nature 447, 72–75 (2007). https://doi.org/10.1038/nature05761
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature05761
This article is cited by
-
Advancements in the fabrication and characterization of actinide targets for superheavy element production
Journal of Radioanalytical and Nuclear Chemistry (2023)
-
The periodic table and the physics that drives it
Nature Reviews Chemistry (2020)
-
Transactinide studies with sulfur macrocyclic extractant using mercury
Journal of Radioanalytical and Nuclear Chemistry (2020)
-
Ion Mobilities for Heaviest Element Identification
Hyperfine Interactions (2020)
-
The current status of the MASHA setup
Hyperfine Interactions (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.