Abstract
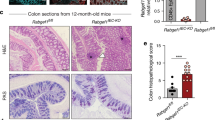
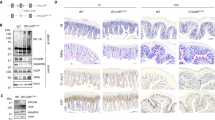
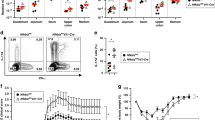
Deregulation of intestinal immune responses seems to have a principal function in the pathogenesis of inflammatory bowel disease1,2,3,4. The gut epithelium is critically involved in the maintenance of intestinal immune homeostasis—acting as a physical barrier separating luminal bacteria and immune cells, and also expressing antimicrobial peptides3,5,6. However, the molecular mechanisms that control this function of gut epithelial cells are poorly understood. Here we show that the transcription factor NF-κB, a master regulator of pro-inflammatory responses7,8, functions in gut epithelial cells to control epithelial integrity and the interaction between the mucosal immune system and gut microflora. Intestinal epithelial-cell-specific inhibition of NF-κB through conditional ablation of NEMO (also called IκB kinase-γ (IKKγ)) or both IKK1 (IKKα) and IKK2 (IKKβ)—IKK subunits essential for NF-κB activation7,8,9—spontaneously caused severe chronic intestinal inflammation in mice. NF-κB deficiency led to apoptosis of colonic epithelial cells, impaired expression of antimicrobial peptides and translocation of bacteria into the mucosa. Concurrently, this epithelial defect triggered a chronic inflammatory response in the colon, initially dominated by innate immune cells but later also involving T lymphocytes. Deficiency of the gene encoding the adaptor protein MyD88 prevented the development of intestinal inflammation, demonstrating that Toll-like receptor activation by intestinal bacteria is essential for disease pathogenesis in this mouse model. Furthermore, NEMO deficiency sensitized epithelial cells to tumour-necrosis factor (TNF)-induced apoptosis, whereas TNF receptor-1 inactivation inhibited intestinal inflammation, demonstrating that TNF receptor-1 signalling is crucial for disease induction. These findings demonstrate that a primary NF-κB signalling defect in intestinal epithelial cells disrupts immune homeostasis in the gastrointestinal tract, causing an inflammatory-bowel-disease-like phenotype. Our results identify NF-κB signalling in the gut epithelium as a critical regulator of epithelial integrity and intestinal immune homeostasis, and have important implications for understanding the mechanisms controlling the pathogenesis of human inflammatory bowel disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Bouma, G. & Strober, W. The immunological and genetic basis of inflammatory bowel disease. Nature Rev. Immunol. 3, 521–533 (2003)
Hanauer, S. B. Inflammatory bowel disease: epidemiology, pathogenesis, and therapeutic opportunities. Inflamm. Bowel Dis. 12, (suppl. 1)S3–S9 (2006)
Macdonald, T. T. & Monteleone, G. Immunity, inflammation, and allergy in the gut. Science 307, 1920–1925 (2005)
Neurath, M. F., Finotto, S. & Glimcher, L. H. The role of Th1/Th2 polarization in mucosal immunity. Nature Med. 8, 567–573 (2002)
Abreu, M. T., Fukata, M. & Arditi, M. TLR signaling in the gut in health and disease. J. Immunol. 174, 4453–4460 (2005)
Wehkamp, J., Fellermann, K., Herrlinger, K. R., Bevins, C. L. & Stange, E. F. Mechanisms of disease: defensins in gastrointestinal diseases. Nature Clin. Pract. Gastroenterol. Hepatol. 2, 406–415 (2005)
Karin, M. & Greten, F. R. NF-κB: linking inflammation and immunity to cancer development and progression. Nature Rev. Immunol. 5, 749–759 (2005)
Li, Q. & Verma, I. M. NF-κB regulation in the immune system. Nature Rev. Immunol. 2, 725–734 (2002)
Ghosh, S. & Karin, M. Missing pieces in the NF-κB puzzle. Cell 109 (suppl.) S81–S96 (2002)
Schmidt-Supprian, M. et al. NEMO/IKKγ-deficient mice model incontinentia pigmenti. Mol. Cell 5, 981–992 (2000)
Madison, B. B. et al. Cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. J. Biol. Chem. 277, 33275–33283 (2002)
Becker, C. et al. TGF-β suppresses tumor progression in colon cancer by inhibition of IL-6 trans-signaling. Immunity 21, 491–501 (2004)
Rudolph, D. et al. Severe liver degeneration and lack of NF-κB activation in NEMO/IKKγ-deficient mice. Genes Dev. 14, 854–862 (2000)
Makris, C. et al. Female mice heterozygous for IKKγ/NEMO deficiencies develop a dermatopathy similar to the human X-linked disorder incontinentia pigmenti. Mol. Cell 5, 969–979 (2000)
Darmoul, D. & Ouellette, A. J. Positional specificity of defensin gene expression reveals Paneth cell heterogeneity in mouse small intestine. Am. J. Physiol. 271, G68–G74 (1996)
Lan, J. G. et al. Different cytokine response of primary colonic epithelial cells to commensal bacteria. World J. Gastroenterol. 11, 3375–3384 (2005)
Fellermann, K. et al. A chromosome 8 gene-cluster polymorphism with low human β-defensin 2 gene copy number predisposes to Crohn disease of the colon. Am. J. Hum. Genet. 79, 439–448 (2006)
Greten, F. R. et al. IKKβ links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 118, 285–296 (2004)
Pasparakis, M. et al. TNF-mediated inflammatory skin disease in mice with epidermis-specific deletion of IKK2. Nature 417, 861–866 (2002)
Li, Q., Estepa, G., Memet, S., Israel, A. & Verma, I. M. Complete lack of NF-κB activity in IKK1 and IKK2 double-deficient mice: additional defect in neurulation. Genes Dev. 14, 1729–1733 (2000)
Gareus, R. et al. Normal epidermal differentiation but impaired skin barrier formation upon keratinocyte-restricted IKK1 ablation. Nature Cell Biol. doi:10.1038/ncb1560 (in the press).
Kawai, T. & Akira, S. TLR signaling. Cell Death Differ. 13, 816–825 (2006)
Targan, S. R. et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor α for Crohn’s disease. Crohn’s Disease cA2 Study Group. N. Engl. J. Med. 337, 1029–1035 (1997)
Luedde, T. et al. Deletion of NEMO/IKKγ in liver parenchymal cells causes steatohepatitis and hepatocellular carcinoma. Cancer Cell 11, 119–132 (2007)
Pasparakis, M., Alexopoulou, L., Episkopou, V. & Kollias, G. Immune and inflammatory responses in TNFα-deficient mice: a critical requirement for TNFα in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J. Exp. Med. 184, 1397–1411 (1996)
Pfeffer, K. et al. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell 73, 457–467 (1993)
Kawai, T., Adachi, O., Ogawa, T., Takeda, K. & Akira, S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity 11, 115–122 (1999)
Becker, C. et al. Constitutive p40 promoter activation and IL-23 production in the terminal ileum mediated by dendritic cells. J. Clin. Invest. 112, 693–706 (2003)
Becker, C. et al. In vivo imaging of colitis and colon cancer development in mice using high resolution chromoendoscopy. Gut 54, 950–954 (2005)
Acknowledgements
We thank K. Pfeffer and S. Akira for providing TNFRI-deficient and MyD88-deficient mice, respectively. This work was supported by grants from the German Research Council to C.B. and M.F.N., and by EU-FP6 grants MUGEN and IMDEMI to M.P.; A.W. received a research fellowship from the Alexander von Humboldt Foundation.
Author Contributions A. Nenci, C.B., A.W., R.G, G.v.L, M.F.N and M.P. designed the research. A. Nenci, C.B, A.W, R.G, G.v.L, S.D., M.H., A. Nikolaev and C.N. performed the research. B.M. and D.G. contributed new reagents. A. Nenci, C.B., A.W., R.G, M.F.N and M.P. analysed the data and wrote the paper. M.F.N. and M.P share senior authorship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-8 with Legends and Supplementary Methods. (PDF 4265 kb)
Rights and permissions
About this article
Cite this article
Nenci, A., Becker, C., Wullaert, A. et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature 446, 557–561 (2007). https://doi.org/10.1038/nature05698
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature05698
This article is cited by
-
IKKε and TBK1 prevent RIPK1 dependent and independent inflammation
Nature Communications (2024)
-
Butyricimonas is a key gut microbiome component for predicting postoperative recurrence of esophageal cancer
Cancer Immunology, Immunotherapy (2024)
-
Alterations in the human oral microbiota in systemic lupus erythematosus
Journal of Translational Medicine (2023)
-
OTULIN protects the intestinal epithelium from apoptosis during inflammation and infection
Cell Death & Disease (2023)
-
Inner mitochondrial membrane protein Prohibitin 1 mediates Nix-induced, Parkin-independent mitophagy
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.