Abstract
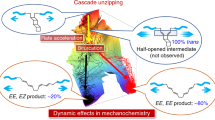
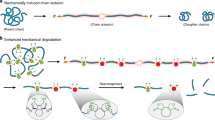
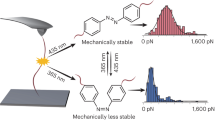
During the course of chemical reactions, reactant molecules need to surmount an energy barrier to allow their transformation into products. The energy needed for this process is usually provided by heat, light, pressure or electrical potential, which act either by changing the distribution of the reactants on their ground-state potential energy surface or by moving them onto an excited-state potential energy surface and thereby facilitate movement over the energy barrier. A fundamentally different way of initiating or accelerating a reaction is the use of force to deform reacting molecules along a specific direction of the reaction coordinate. Mechanical force has indeed been shown to activate covalent bonds in polymers, but the usual result is chain scission1. Here we show that mechanically sensitive chemical groups make it possible to harness the mechanical forces generated when exposing polymer solutions to ultrasound2, and that this allows us to accelerate rearrangement reactions and bias reaction pathways to yield products not obtainable from purely thermal or light-induced reactions. We find that when placed within long polymer strands, the trans and cis isomers of a 1,2-disubstituted benzocyclobutene undergo an ultrasound-induced electrocyclic ring opening in a formally conrotatory and formally disrotatory process, respectively, that yield identical products. This contrasts with reaction initiation by light or heat alone3, in which case the isomers follow mutually exclusive pathways to different products. Mechanical forces associated with ultrasound can thus clearly alter the shape of potential energy surfaces4 so that otherwise forbidden or slow processes proceed under mild conditions, with the directionally specific nature of mechanical forces providing a reaction control that is fundamentally different from that achieved by adjusting chemical or physical parameters. Because rearrangement in our system occurs before chain scission, the effect we describe might allow the development of materials that are activated by mechanical stress fields.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Beyer, M. K. & Clausen-Schaumann, H. Mechanochemistry: The mechanical activation of covalent bonds. Chem. Rev. 105, 2921–2948 (2005)
Basedow, A. M. & Ebert, K. H. Ultrasonic degradation of polymers in solution. Adv. Polym. Sci. 22, 83–148 (1977)
Woodward, R. B. & Hoffmann, R. The conservation of orbital symmetry. Angew. Chem. Int. Edn Engl. 8, 781–853 (1969)
Evans, E. Probing the relation between force-lifetime and chemistry in single molecular bonds. Annu. Rev. Biophys. Biomol. Struct. 30, 105–128 (2001)
Beyer, M. K. The mechanical strength of a covalent bond calculated by density functional theory. J. Chem. Phys. 112, 7307–7312 (2000)
Roth, W. R., Rekowski, V., Börner, S. & Quast, M. Die disrotative cyclobuten-ringöffnung. Liebigs Ann. Chem. 409–430 (1996)
Boffa, L. S. & Novak, B. M. “Link-functionalized” polymers: an unusual macromolecular architecture through bifunctional initiation. Macromolecules 30, 3494–3506 (1997)
Nguyen, T. Q. & Kausch, H.-H. Mechanochemical degradation in transient elongational flow. Adv. Polym. Sci. 100, 73–182 (1992)
Berkowski, K. L., Potisek, S. L., Hickenboth, C. R. & Moore, J. S. Ultrasound-induced site-specific cleavage of azo-functionalized poly(ethylene glycol). Macromolecules 38, 8975–8978 (2005)
Reddy, P. Y., Kondo, S., Fujita, S. & Toru, T. Efficient synthesis of fluorophore-linked maleimide derivatives. Synthesis 999–1002 (1998)
Price, G. J. The use of ultrasound for the controlled degradation of polymer solutions. Adv. Sonochem. 1, 231–287 (1990)
Arnold, B. J., Sammes, P. G. & Wallace, T. W. Photochemical reactions. Part IV. Thermal generation of photoenols and their derivatives from disubstituted 1,2-dihydrobenzocyclobutenes. J. Chem. Soc. Perkin Trans. I 415 (1974)
Suslick, K. S., Goodale, J. W., Schubert, P. F. & Wang, H. H. Sonochemistry and sonocatalysis of metal carbonyls. J. Am. Chem. Soc. 105, 5781–5785 (1983)
Graessley, W. W. Polymer chain dimensions and the dependence of viscoelastic properties on concentration, molecular weight and solvent power. Polymer 21, 258–262 (1980)
Rodd, L. E., Scott, T. P., Boger, D. V., Cooper-White, J. J. & McKinley, G. H. The inertio-elastic planar entry flow of low-viscosity elastic fluids in micro-fabricated geometries. J. Non-Newtonian Fluid Mech. 129, 1–22 (2005)
Kersey, F. R., Yount, W. C. & Craig, S. L. Single-molecule force spectroscopy of bimolecular reactions: system homology in the mechanical activation of ligand substitution reactions. J. Am. Chem. Soc. 128, 3886–3887 (2006)
Acknowledgements
We thank T. Martínez, K. Suslick and P. Beak for discussions. This work was supported by grants from the Air Force Office of Scientific Research and The Petroleum Research Fund.
Author Contributions J.S.M. conceived the BCB experiment. C.R.H. performed all experiments and initial computational analyses. J.B. performed the final computational analysis. S.R. Wilson performed the crystallography. N.R.S., S.R. White and J.S.M. directed the research and all authors wrote the manuscript.
The atomic coordinates for cis-1,2-bis[(3-carboxypropanoyl)oxy]-1,2-dihydrobenzocyclobutene 2 (accession number 628670), N-(1-pyrene)-2,3-naphthimide (accession number 628377), and 4,9-diacetoxy-2-(1-pyrenyl)-(3aR,4c,9c,9ac)-3a,4,9,9a-tetrahydro-benzo[f]isoindole-1,3-dione 6 (accession number 628671) have been deposited in the Cambridge Crystal Structure Database.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-10 with Legends, Supplementary discussion and additional references. The file contains all of the supporting information including results of computational studies, results of all control experiments, procedures for the synthesis and characterization of all new compounds reported in the manuscript, representative GPC traces of ultrasound experiments, and 13C NMR spectra of all new reported compounds. (PDF 1266 kb)
Supplementary Video Streaming
(HTML 3370 kb)
Rights and permissions
About this article
Cite this article
Hickenboth, C., Moore, J., White, S. et al. Biasing reaction pathways with mechanical force. Nature 446, 423–427 (2007). https://doi.org/10.1038/nature05681
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature05681
This article is cited by
-
Editing of polymer backbones
Nature Reviews Chemistry (2023)
-
Mechanochemical synthesis of an elusive fluorinated polyacetylene
Nature Chemistry (2021)
-
Distal conformational locks on ferrocene mechanophores guide reaction pathways for increased mechanochemical reactivity
Nature Chemistry (2021)
-
Mechanochemistry of phosphate esters confined between sliding iron surfaces
Communications Chemistry (2021)
-
Sub-molecular structural relaxation at a physisorbed interface with monolayer organic single-crystal semiconductors
Communications Physics (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.