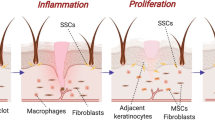
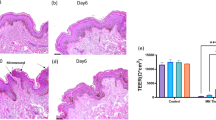
Abstract
Tissue-engineered skin is now a reality. For patients with extensive full-thickness burns, laboratory expansion of skin cells to achieve barrier function can make the difference between life and death, and it was this acute need that drove the initiation of tissue engineering in the 1980s. A much larger group of patients have ulcers resistant to conventional healing, and treatments using cultured skin cells have been devised to restart the wound-healing process. In the laboratory, the use of tissue-engineered skin provides insight into the behaviour of skin cells in healthy skin and in diseases such as vitiligo, melanoma, psoriasis and blistering disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Herndon, D. N. et al. A comparison of conservative versus early excision. Therapies in severely burned patients. Ann. Surg. 209, 547–552 (1989).
Burn Incidence and Treatment in the United States: 1999 Fact Sheet (The Burn Foundation, Philadelphia, 1999).
Rose, J. K. & Herndon, D. N. Advances in the treatment of burn patients. Burns 23 (suppl. 1), S19–S26 (1997).
Falanga, V. Chronic wounds: pathophysiologic and experimental considerations. J. Invest. Dermatol. 100, 721–725 (1993).
Phillips, T., Stanton, B., Provan, A. & Lew, R. A study of the impact of leg ulcers on quality of life: financial, social, and psychologic implications. J. Am. Acad. Dermatol. 31, 49–53 (1994).
Bittencourt, F. V. et al. Large congenital melanocytic nevi and the risk of development of malignant melanoma and neurocutaneous melanocytosis. Pediatrics 106, 736–741 (2000).
Cooper, M. L., Spielvogel, R. L., Hansbrough, J. F., Boyce, S. T. & Frank, D. H. Reconstitution of the histologic characteristics of a giant congenital nevomelanocytic nevus employing the athymic mouse and a cultured skin substitute. J. Invest. Dermatol. 97, 649–658 (1991).
Gallico, G. G. et al. Cultured epithelial autografts for giant congenital nevi. Plast. Reconstr. Surg. 84, 1–9 (1989).
Rheinwald, J. G. & Green, H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell 6, 331–343 (1975).
Rheinwald, J. G. & Green, H. Epidermal growth factor and the multiplication of cultured human epidermal keratinocytes. Nature 265, 421–424 (1977)
Green, H., Kehinde, O. & Thomas, J. Growth of cultured human epidermal cells into multiple epithelia suitable for grafting. Proc. Natl Acad. Sci. USA 76, 5665–5668 (1979).
O'Connor, N. E., Mulliken, J. B., Banks-Schlegel, S., Kehinde, O. & Green, H. Grafting of burns with cultured epithelium prepared from autologous epidermal cells. Lancet i, 75–78 (1981).
Gallico, G. G., O'Connor, N. E., Compton, C. C., Kehinde, O. & Green, H. Permanent coverage of large burn wounds with autologous cultured human epithelium. New Engl J. Med. 311, 448–451 (1984).
Barrandon, Y. & Green, H. Three clonal types of keratinocyte with different capacities for multiplication. Proc. Natl Acad. Sci. USA 84, 2302–2306 (1987).
Fuchs, E. Epidermal differentiation: the bare essentials. J. Cell Biol. 111, 2807–2814 (1990).
Gambardella, L. & Barrandon, Y. The multifaceted adult epidermal stem cell. Curr. Opin. Cell Biol. 15, 771–777 (2003).
Cuono, C., Langdon, R. & McGuire, J. Use of cultured epidermal autografts and dermal allografts as skin replacement after burn injury. Lancet i, 1123–1124 (1986).
Herndon, D. N. & Parks, D. H. Comparison of serial debridement and autografting and early massive excision with cadaver skin overlay in the treatment of large burns in children. J. Trauma 26, 149–152 (1986).
Burke, J. F., Yannas, I. V., Quinby, W. C., Bondoc, C. C. & Jung, W. K. Successful use of a physiologically acceptable artificial skin in the treatment of extensive burn injury. Ann. Surg. 194, 413–428 (1981).
Huang, Q., Dawson, R. A., Pegg, D. E., Kearney, J. N. & MacNeil, S. Use of peracetic acid to sterilise human donor skin for production of acellular matrices for clinical use. Wound Repair Regen. 12, 276–287 (2004).
Stern, R., McPherson, M. & Longaker, M. T. Histologic study of artificial skin used in the treatment of full thickness thermal injury. J. Burn Care Rehabil. 11, 7–13 (1990).
Boyce, S. T. et al. Comparative assessment of cultured skin substitutes and native skin autograft for treatment of full-thickness burns. Ann. Surg. 222, 743–752 (1995).
Boyce, S. T. et al. The 1999 Clinical Research Award. Cultured skin substitutes combined with Integra artificial skin to replace native skin autograft and allograft for closure of full-thickness burns. J. Burn Care Rehabil. 20, 453–461 (1999).
Boyce, S. T. et al. Cultured skin substitutes reduce donor skin harvesting for closure of excised, full-thickness burns. Ann. Surg. 235, 269–279 (2002).
Supp, D. M. & Boyce, S. T. Engineered skin substitutes: practices and potentials. Clin. Dermatol. 23, 403–412 (2005).
Iuchi, S., Dabelsteen, S., Easley, K., Rheinwald, J. G. & Green, H. Immortalized keratinocyte lines derived from human embryonic stem cells. Proc. Natl Acad. Sci. USA 103, 1792–1797 (2006).
McPherson, T. B. et al. Galα(1,3)Gal epitope in porcine small intestinal submucosa. Tissue Eng. 6, 233–239 (2000).
Marston, W. A., Hanft, J., Norwood, P. & Pollak, R. The efficacy and safety of Dermagraft in improving the healing of chronic diabetic foot ulcers: results of a prospective randomized trial. Diabetes Care 26, 1701–1705 (2003).
Kumar, R. J., Kimble, R. M., Boots, R. & Pegg, S. P. Treatment of partial-thickness burns: a prospective randomized trial using Transcyte™. ANZ J. Surg. 74, 622–626 (2004).
Bello, Y. M. & Falabella, A. F. The role of Graftskin (Apligraf®) in difficult-to-heal venous leg ulcers. J. Wound Care 11, 182–183 (2003).
Lipkin, S., Chaikof, E., Isseroff, Z. & Silverstein, P. Effectiveness of bilayered cellular matrix in healing of neuropathic diabetic foot ulcers: results of a multicenter pilot trial. Wounds 15, 230–236 (2003).
Carver, N., Navsaria, H., Green, C. J. & Leigh, I. M. Acute rejection of cultured keratinocyte allografts in nonimmunosuppressed pigs. Transplantation 52, 918–921 (1991).
Brain, A. et al. Survival of cultured allogeneic keratinocytes transplanted to deep dermal bed assessed with probe specific for Y chromosome. Br. Med. J. 298, 917–919 (1989).
Hermans, M. H. Clinical experience with glycerol-preserved donor skin treatment in partial thickness burns. Burns Incl. Therm. Inj. 15, 57–59 (1989).
Wainwright, D. et al. Clinical evaluation of an acellular allograft dermal matrix in full-thickness burns. J. Burn Care Rehabil. 17, 124–136 (1996).
Jarman-Smith, M. L. et al. Porcine collagen crosslinking, degradation and its capability for fibroblast adhesion and proliferation. J. Mater. Sci. Mater. Med. 15, 925–932 (2004).
Boyce, S. T. et al. Cultured skin substitutes reduce requirements for harvesting of skin autograft for closure of excised, full-thickness burns. J. Trauma 60, 821–829 (2006).
Ralston, D. R. et al. The requirement for basement membrane antigens in the production of human epidermal/dermal composites in vitro. Br. J. Dermatol. 140, 605–615 (1999).
Sahota, P. S. et al. Development of a reconstructed human skin model for angiogenesis. Wound Repair Regen. 11, 275–284 (2003).
Medalie, D. A. et al. Differences in dermal analogs influence subsequent pigmentation, epidermal differentiation, basement membrane and rete ridge formation of transplanted composite skin grafts. Transplantation 64, 454–465 (1997).
Swope, V. B., Supp, A. P., Schwemberger, S., Babcock, G. & Boyce, S. Increased expression of integrins and decreased apoptosis correlate with increased melanocyte retention in cultured skin substitutes. Pigment Cell Res. 19, 424–433 (2006).
Ratner, B. D. & Bryant, S. J. Biomaterials: where we have been and where we are going. Annu. Rev. Biomed. Eng. 6, 41–75 (2004).
Wright, K. A. et al. Alternative delivery of keratinocytes using a polyurethane membrane and the implications for its use in the treatment of full-thickness burn injury. Burns 24, 7–17 (1998).
Horch, R. E., Bannasch, H. & Stark, G. B. Transplantation of cultured autologous keratinocytes in fibrin sealant biomatrix to resurface chronic wounds. Transplant Proc. 33, 642–644 (2001).
Navarro, F. A. et al. Sprayed keratinocyte suspensions accelerate epidermal coverage in a porcine microwound model. J. Burn Care Rehabil. 21, 513–518 (2000).
Horch, R. E., Debus, M., Wagner, G. & Stark, G. B. Cultured human keratinocytes on type I collagen membranes to reconstitute the epidermis. Tissue Eng. 6, 53–67 (2000).
Haddow, D. B. et al. Plasma-polymerized surfaces for culture of human keratinocytes and transfer of cells to an in vitro wound-bed model. J. Biomed. Mater. Res. A 64, 80–87 (2003).
Tausche, A. K. et al. An autologous epidermal equivalent tissue-engineered from follicular outer root sheath keratincoytes is as effective as split-thickness skin autograft in recalcitrant vascular leg ulcers. Wound Repair Regen. 11, 248–252 (2003).
Currie, L. J., Martin, R., Sharpe, J. R. & James, S. E. A comparison of keratinocyte cell sprays with and without fibrin glue. Burns 29, 677–685 (2003).
Hernon, C. A. et al. Clinical experience using cultured epithelial autografts lead to an alternative methodology for transferring skin cells from the laboratory to the patient. Regen. Med. 1, 809–821 (2006).
Moustafa, M. et al. A new autologous keratinocyte dressing treatment for non-healing diabetic neuropathic foot ulcers. Diabet. Med. 21, 786–789 (2004).
Zhu, N. et al. Treatment of burns and chronic wounds using a new cell transfer dressing for delivery of autologous keratinocytes. Eur. J. Plast. Surg. 28, 319–330 (2005).
France, R. M., Short, R. D., Dawson, R. A. & MacNeil, S. Attachment of human keratinocytes to plasma co-polymers of acrylic acid/octa-1,7-diene and allyl amine/octa-1,7-diene. J. Mater. Chem. 8, 37–42 (1998).
De Luca, M. et al. Multicentre experience in the treatment of burns with autologous and allogeneic cultured epithelium, fresh or preserved in a frozen state. Burns 15, 303–309 (1989).
Sun, T. et al. Self-organisation of skin cells in three-dimensional electrospun polystyrene scaffolds. Tissue Eng. 11, 1023–1033 (2005).
Sun, T., Jackson, S., Haycock, J. W. & MacNeil, S. Culture of skin cells in 3D rather than 2D improves their ability to survive exposure to cytotoxic agents. J. Biotechnol. 122, 372–381 (2006).
Sauer, U. G., Spielmann, H. & Rusche, B. Fourth EU report on the statistics on the number of animals used for scientific purposes in 2002 — trends, problems, conclusions. ALTEX 22, 59–67 (2002).
Facy, V., Flouret, V., Regnier, M. & Schmidt, R. Reactivity of Langerhans cells in human reconstructed epidermis to known allergens and UV radiation. Toxicol. In Vitro 19, 787–795 (2005).
Welss, T., Basketter, D. A. & Schroder, K. R. In vitro skin irritation: facts and future. State of the art review of mechanisms and models. Toxicol. In Vitro 18, 231–243 (2004).
Netzlaff, F., Lehr, C. M., Wertz, P. W. & Schaefer, U. F. The human epidermis models EpiSkin, SkinEthic and EpiDerm: an evaluation of morphology and their suitability for testing phototoxicity, irritancy, corrosivity, and substance transport. Eur. J. Pharm. Biopharm. 60, 167–178 (2005).
Kandarova, H. et al. Assessment of the skin irritation potential of chemicals by using the SkinEthic reconstructed human epidermal model and the common skin irritation protocol evaluated in the ECVAM skin irritation validation study. Altern. Lab. Anim. 34, 393–406 (2006).
Bessou, S. et al. Ex vivo study of skin phototypes. J. Invest. Dermatol. 107, 684–688 (1996).
Hedley, S. J. et al. Fibroblasts play a regulatory role in the control of pigmentation in reconstructed human skin from skin types I and II. Pigment Cell Res. 15, 49–56 (2002).
Cario-Andre, M., Pain, C., Gauthier, Y., Casoli, V. & Taieb, A. In vivo and in vitro evidence of dermal fibroblasts influence on human epidermal pigmentation. Pigment Cell Res. 19, 434–442 (2006).
Meier, F., Nesbit, M. & Hsu, M.-Y. Human melanoma progression in skin reconstructs: biological significance of bFGF. Am. J. Pathol. 156, 193–200 (2000).
Eves, P. et al. Characterization of an in vitro model of human melanoma invasion based on reconstructed human skin. Br. J. Dermatol. 142, 210–222 (2003).
Eves, P. et al. Melanoma invasion in reconstructed human skin is influenced by skin cells — investigation of the role of proteolytic enzymes. Clin. Exp. Metastasis 20, 685–700 (2003).
Eves, P. et al. Anti-inflammatory and anti-invasive effects of alpha-melanocyte-stimulating hormone in human melanoma cells. Br. J. Cancer 89, 2004–2015 (2003).
Ralston, D. R. et al. Keratinocytes contract normal human dermal extracellular matrix and reduce soluble fibronectin production by fibroblasts in a skin composite model. Br. J. Plast. Surg. 50, 408–415 (1997).
Chakrabarty, K. H. et al. Keratinocyte-driven contraction of reconstructed human skin. Wound Repair Regen. 9, 95–106 (2001).
Harrison, C. A. et al. Use of an in vitro model of tissue-engineered human skin to investigate the mechanism of skin graft contraction. Tissue Eng. 1 Oct 2006 [Epub ahead of print].
Souren, J. M., Ponec, M. & Van Wijk, R. Contraction of collagen by human fibroblasts and keratinocytes. In Vitro Cell Dev. Biol. 25, 1039–1045 (1989).
Grinnell, F. Fibroblasts, myofIbroblasts and wound contraction. J. Cell Biol. 124, 401–404 (1994).
Barker, C. L. et al. The development and characterization of an in vitro model of psoriasis. J. Invest. Dermatol. 123, 892–901 (2004).
Ferrari, S., Pellegrini, G., Matsui, T., Mavilio, F. & De Luca, M. Gene therapy in combination with tissue engineering to treat epidermolysis bullosa. Expert Opin. Biol. Ther. 6, 367–378 (2006).
Shah, M. et al. Role of elevated plasma transforming growth factor β1 levels in wound healing. Am. J. Pathol. 154, 1115–1124 (1989)
O'Kane, S. & Ferguson, M. W. Transforming growth factor βs and wound healing. Int. J. Biochem. Cell Biol. 29, 63–78 (1997).
Ferguson, M. W. & O'Kane, S. Scar-free healing: from embryonic mechanisms to adult therapeutic intervention. Phil. Trans. R. Soc. Lond. B 359, 839–850 (2004).
Shier, D., Butler, J. & Lewis, R. in Hole's Human Anatomy and Physiology 8th Edn 160–183 (McGraw Hill, 1999).
Herndon, D. N. & Parks, D. H. Comparison of serial debridement and autografting and early massive excision with cadaver skin overlay in the treatment of large burns in children. J. Trauma 26, 149–152 (1986).
Delvoye, P. et al. Fibroblasts induce the assembly of the macromolecules of the basement membrane. J. Invest. Dermatol. 90, 276–282 (1990).
Konig, A. & Brucker-Tuderman, L. Epithelial–mesenchymal interactions enhance expression of collagen VII in vitro. J. Invest. Dermatol. 96, 803–808 (1991).
Demarchez, M., Hartmann, D. J., Regnier, M. & Asselineau, D. The role of fibroblasts in dermal vascularization and remodeling of reconstructed human skin after transplantation onto the nude mouse. Transplantation 54, 317–326 (1992).
Acknowledgements
The author regrets that space restrictions preclude full reference to all those who have contributed to this field. Sheila MacNeil's research is supported by grants from EPSRC, BBSRC and the Wellcome Trust.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Sheila MacNeil is a director of CellTran, which has developed a wound-healing product known as Myskin.
Rights and permissions
About this article
Cite this article
MacNeil, S. Progress and opportunities for tissue-engineered skin. Nature 445, 874–880 (2007). https://doi.org/10.1038/nature05664
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature05664
This article is cited by
-
All-in-one smart dressing for simultaneous angiogenesis and neural regeneration
Journal of Nanobiotechnology (2023)
-
CD146 expression profile in human skin and pre-vascularized dermo-epidermal skin substitutes in vivo
Journal of Biological Engineering (2023)
-
Modeling of three-dimensional innervated epidermal like-layer in a microfluidic chip-based coculture system
Nature Communications (2023)
-
Development of a tissue-engineered skin model with epidermal, dermal and hypodermal components
In vitro models (2023)
-
Natural fiber biocomposites via 4D printing technologies: a review of possibilities for agricultural bio-mulching and related sustainable applications
Progress in Additive Manufacturing (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.