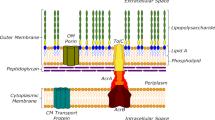
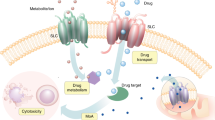
Abstract
The acquisition of multidrug resistance is a serious impediment to improved healthcare. Multidrug resistance is most frequently due to active transporters that pump a broad spectrum of chemically distinct, cytotoxic molecules out of cells, including antibiotics, antimalarials, herbicides and cancer chemotherapeutics in humans. The paradigm multidrug transporter, mammalian P-glycoprotein, was identified 30 years ago. Nonetheless, success in overcoming or circumventing multidrug resistance in a clinical setting has been modest. Recent structural and biochemical data for several multidrug transporters now provide mechanistic insights into how they work. Organisms have evolved several elegant solutions to ridding the cell of such cytotoxic compounds. Answers are emerging to questions such as how multispecificity for different drugs is achieved, why multidrug resistance arises so readily, and what chance there is of devising a clinical solution.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Schinkel, A. H., Wagenaar, E., Mol, C. A. & van Deemter, L. P-glycoprotein in the blood-brain barrier of mice influences the brain penetration and pharmacological activity of many drugs. J. Clin. Invest. 97, 2517–2524 (1996)
Saier, M. H. & Paulsen, I. T. Phylogeny of multidrug transporters. Semin. Cell Dev. Biol. 12, 205–213 (2001)
Higgins, C. F. ABC transporters: from microorganisms to man. Annu. Rev. Cell Biol. 8, 67–113 (1992)
Juliano, R. L. & Ling, V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim. Biophys. Acta 455, 152–162 (1976)
Gottesman, M. M. Fojo, T. & Bates, S. E. Multidrug resistance in cancer: role of ATP-dependent transporters. Nature Rev. Cancer 2, 48–58 (2001)
Holland, I. B., Cole, S. P. C., Kuchler, K., Higgins, C. F. (eds) ABC Proteins: from Bacteria to Man (Academic, London, 2003)
van Veen, H. W. et al. A bacterial antibiotic resistance gene that complements the human multidrug resistance P-glycoprotein gene. Nature 391, 291–295 (1998)
Higgins, C. F. et al. A family of related ATP-binding subunits coupled to many distinct biological processes in bacteria. Nature 323, 448–450 (1986)
Rosenberg, M. F., Callaghan, R., Ford, R. C. & Higgins, C. F. Structure of the multidrug-resistance P-glycoprotein to 2.5 nm resolution determined by electron microscopy. J. Biol. Chem. 272, 10685–10694 (1997)
Rosenberg, M. F. et al. Repacking of the transmembrane domains of P-glycoprotein, during the transport ATPase cycle. EMBO J. 20, 5615–5625 (2001)
Rosenberg, M. F., Kamis, A. B., Callaghan, R., Higgins, C. F. & Ford, R. C. Three-dimensional structures of the mammalian multidrug resistance P-glycoprotein demonstrate major conformational changes in the transmembrane domains upon nucleotide binding. J. Biol. Chem. 278, 8294–8299 (2003)
Rosenberg, M. F., Callaghan, R., Modok, S., Higgins, C. F. & Ford, R. C. Three-dimensional structure of P-glycoprotein: the transmembrane regions adopt an asymmetric configuration in the nucleotide-bound state. J. Biol. Chem. 280, 2857–2862 (2005)
Dawson, R. J. P. & Locher, K. P. Structure of a bacterial multidrug ABC transporter. Nature 443, 180–185 (2006)
Loo, T. W. & Clarke, D. M. The packing of the transmembrane segments of human multidrug resistance P-glycoprotein is revealed by disulfide cross-linking analysis. J. Biol. Chem. 275, 5253–5256 (2000)
Stenham, D. R. et al. An atomic detail model for the human ATP-binding cassette transporter, P-glycoprotein, derived from disulphide cross-linking and homology modelling. FASEB J. 17, 2287–2289 (2003)
Smith, P. C. et al. ATP binding to the motor domain from an ABC transporter drives formation of a nucleotide sandwich dimer. Mol. Cell 10, 139–149 (2002)
Locher, K. P., Lee, A. T. & Rees, D. C. The E. coli BtuCD structure: a framework for ABC transporter architecture and mechanism. Science 296, 1091–1098 (2002)
Pinkett, H. W., Lee, A. T., Lum, P., Locher, K. P. & Rees, D. C. An inward facing conformation of a putative metal-chelate-type ABC transporter. Science 315, 373–377 (2007); published online 7 December 2006.
Chang, G. et al. Retraction. Science 314, 1875 (2006)
Zolnerciks, J. K., Wooding, C. & Linton, K. J. In vivo evidence for a Sav1866-like architecture for the human multidrug transporter P-glycoprotein. J. Biol. Chem. (submitted).
Higgins, C. F. & Linton, K. J. The ATP switch model for ABC transporters. Nature Struct. Mol. Biol. 11, 918–926 (2004)
Dong, J., Yang, G. & Mchaourab, H. S. Structural basis for energy transduction in the transport cycle of MsbA. Science 308, 1023–1028 (2005)
Dey, S., Ramachandra, M., Pastan, I., Gottesman, M. M. & Ambudkar, S. V. Evidence for two non-identical drug-interaction sites in the human P-glycoprotein. Proc. Natl Acad. Sci. USA 94, 10594–10599 (1997)
Martin, C. et al. Drug binding sites on P-glycoprotein are altered by ATP binding prior to nucleotide hydrolysis. Biochemistry 39, 11901–11906 (2000)
Martin, C., Higgins, C. F. & Callaghan, R. The vinblastine binding site adopts high- and low-affinity conformations during a transport cycle of P-glycoprotein. Biochemistry 40, 15733–15742 (2001)
Payen, L. F., Gao, M., Westlake, C. J., Cole, S. P. C. & Deeley, R. G. Role of carboxylate residues adjacent to the conserved core walker B motifs in the catalytic cycle of multidrug resistance protein 1 (ABCC1). J. Biol. Chem. 278, 38537–38547 (2003)
van Veen, H. W., Margolles, A., Muller, M., Higgins, C. F. & Konings, W. N. The homodimeric ATP binding cassette transporter LmrA mediates multidrug resistance by a two-site (two-cylinder engine) mechanism. EMBO J. 19, 2503–2514 (2000)
Vergani, P., Lockless, S. W., Nairn, A. C. & Gadsby, D. C. CFTR channel opening by ATP-driven tight dimerization of its nucleotide binding domains. Nature 433, 876–880 (2005)
Higgins, C. F. & Gottesman, M. M. Is the multidrug transporter a flippase? Trends Biochem. Sci. 17, 18–21 (1992)
Bolhuis, H. et al. Multidrug resistance in Lactococcus lactis: evidence for ATP-dependent drug extrusion from the inner leaflet of the cytoplasmic membrane. EMBO J. 15, 4239–4245 (1996)
Loo, T. W. & Clarke, D. M. Recent progress in understanding the mechanism of P-glycoprotein-mediated drug efflux. J. Membr. Biol. 206, 173–185 (2005)
Martin, C. et al. Communication between multiple drug binding sites on P-glycoprotein. Mol. Pharmacol. 58, 624–632 (2000)
Shapiro, A. B. & Ling, V. Positively cooperative sites for drug transport by P-glycoprotein with distinct drug specificities. Eur. J. Biochem. 250, 130–137 (1997)
Zelcer, N. et al. Evidence for two interacting ligand binding sites in human multidrug resistance protein 2 (ABCC2). J. Biol. Chem. 278, 23538–23544 (2003)
Lugo, M. R. & Sharom, F. J. Interaction of LDS-751 and rhodamine 123 with P-glycoprotein: evidence for simultaneous binding of both drugs. Biochemistry 44, 14020–14029 (2005)
Ko, D. C., Gordon, M. D., Jin, J. Y. & Scott, M. P. Dynamic movements of organelles containing Niemann-Pick C1 protein: NPC1 involvement in late endocytic events. Mol. Biol. Cell 12, 601–614 (2001)
Ma, Y. et al. Hedgehog-medicated patterning of the mammalian embryo requires transporter-like function of dispatched. Cell 111, 63–75 (2002)
Zgurskaya, H. I. & Nikaido, H. Bypassing the periplasm: reconstitution of the AcrAB multidrug efflux pump of Escherichia coli.. Proc. Natl Acad. Sci. USA 96, 7190–7195 (1999)
Murakami, S., Nakashima, R., Yamashita, E. & Yamaguchi, A. Crystal structure of bacterial multidrug efflux transporter AcrB. Nature 419, 587–593 (2002)
Murakami, S., Nakashima, R., Yamashita, E., Matsumoto, T. & Yamaguchi, A. Crystal structure of a multidrug transporter reveals a functionally rotating mechanism. Nature 443, 173–179 (2006)
Yu, E. W., McDermott, G., Zgurskaya, H. I., Nikaido, H. & Koshland, D. E. Structural basis of multiple drug-binding capacity of the AcrB multidrug efflux pump. Science 300, 976–980 (2003)
Seeger, M. A. et al. Structural asymmetry of AcrB trimer suggests a peristaltic pump mechanism. Science 313, 1295–1298 (2006)
Koronakis, V., Sharff, A., Koronakis, E., Luisi, B. & Hughes, C. Crystal structure of the bacterial membrane protein TolC central to multidrug efflux and protein export. Nature 405, 914–919 (2000)
Aires, J. R. & Nikaido, H. Aminoglycosides are captured from both periplasm and cytoplasm by the AcrD multidrug efflux transporter of Escherichia coli. J. Bacteriol. 187, 1923–1929 (2005)
Schuldiner, S., Lebendiker, M. & Yerushalmi, H. EmrE, the smallest ion-coupled transporter, provides a unique paradigm for structure-function studies. J. Exp. Biol. 200, 335–341 (1997)
Ubarretxena-Belandia, I., Baldwin, J. M., Schuldiner, S. & Tate, C. G. Three-dimensional structure of the bacterial multidrug transporter EmrE shows it is an asymmetric homodimer. EMBO J. 22, 6175–6181 (2003)
Sharoni, M., Steiner-Mordoch, S. & Schuldiner, S. Exploring the binding domain of EmrE, the smallest multidrug transporter. J. Biol. Chem. 280, 32849–32855 (2005)
Soskine, M., Mark, S., Tayer, N., Mizrachi, R. & Schuldiner, S. On parallel and antiparallel topology of a homodimeric multidrug transporter. J. Biol. Chem. 281, 36205–36212 (2006)
Rapp, M., Granseth, E., Seppala, S. & von Heijne, G. Identification and evolution of dual-topology proteins. Nature Struct. Mol. Biol. 13, 112–116 (2006)
Yerushalmi, H. & Schuldiner, S. A model for coupling of H+ and substrate fluxes based on ‘time-sharing’ of a common binding site. Biochemistry 39, 14711–14719 (2000)
Abramson, J. et al. Structure and mechanisms of the lactose permease of Escherichia coli. Science 301, 610–615 (2003)
Huang, Y., Lemieux, M. J., Song, J., Auer, M. & Wang, D. N. Structure and mechanisms of the glycerol-3-phosphate transporter from Escherichia coli. Science 301, 616–620 (2003)
Yin, Y., He, X., Szewczyk, P., Nguyen, T. & Chang, G. Structure of the multidrug transporter EmrD from Escherichia coli. Science 312, 741–744 (2006)
Adler, J. & Bibi, J. Promiscuity in the geometry of electrostatic interactions between the Escherichia coli multidrug resistance transporter MdfA and cationic substrates. J. Biol. Chem. 280, 2721–2729 (2005)
Mazurkiewicz, P., Driessen, A. J. & Konings, W. N. Energetics of wild type and mutant multidrug resistance secondary transporter LmrP of Lactococcus lactis. Biochim. Biophys. Acta 1658, 252–261 (2004)
Putman, M., Koole, L. A., van Veen, H. W. & Konings, W. N. The secondary multidrug transporter LmrP contains multiple drug interaction sites. Biochemistry 38, 13900–13905 (1999)
Lewinson, O., Padan, E. & Bibi, E. Alkalitolerance: a biological function for a multidrug transporter in pH homeostasis. Proc. Natl Acad. Sci. USA 101, 14073–14078 (2004)
Abramson, J., Iwata, S. & Kaback, H. R. Lactose permease as a paradigm for membrane transport proteins (Review). Mol. Membr. Biol. 21, 227–236 (2004)
Zheleznova, E. E., Markham, P. N., Neyfakh, A. A. & Brennan, R. G. Structural basis of multidrug recognition by BmrR, a transcription activator of a multidrug transporter. Cell 96, 353–362 (1999)
Vázquez-Laslop, N., Markham, P. N. & Neyfakh, A. A. Mechanisms of ligand recognition by BmrR, the multidrug-responding transcriptional regulator: mutational analysis of the ligand binding site. Biochemistry 38, 16925–16931 (1999)
Schumacher, M. A. et al. Structural mechanisms of QacR induction and multidrug recognition. Science 294, 2158–2163 (2001)
Schumacher, M. A., Miller, M. C. & Brennan, R. G. Structural mechanisms of the simultaneous binding of two drugs to a multidrug-binding protein. EMBO J. 23, 2923–2930 (2004)
Neyfakh, A. A. Mystery of multidrug transporters: the answer can be simple. Mol. Microbiol. 44, 1123–1130 (2002)
Tame, J. R. H. et al. The structural basis of sequence-independent peptide binding by OppA protein. Science 264, 1578–1581 (1994)
Cupp-Vickery, J., Anderson, R. & Hatziris, Z. Crystal structures of ligand complexes of P450eryF exhibiting homotropic competition. Proc. Natl Acad. Sci. USA 97, 3050–3055 (2000)
Watkins, R. E. et al. The human nuclear xenobiotic receptor PXR: structural determinants of directed promiscuity. Science 292, 2329–2333 (2001)
Ramoni, R. et al. The insect attractant 1-octen-3-ol is the natural ligand of bovine odorant-binding protein. J. Biol. Chem. 276, 7150–7155 (2001)
Chang, G. & Roth, C. B. Structure of MsbA from E. coli: a homolog of the multidrug resistance ATP binding cassette (ABC) transporters. Science 293, 1793–1800 (2001)
Ma, C. & Chang, G. Structure of the multidrug resistance efflux transporter EmrE from Escherichia coli. Proc. Natl Acad. Sci. USA 101, 2852–2857 (2004)
Mitchell, P. A general theory for membrane transport from studies of bacteria. Nature 180, 134–136 (1957)
Smit, J. J. M. et al. Homozygous disruption of the murine mdr2 P-glycoprotein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell 75, 451–462 (1993)
Stein, W. D., Cardarelli, C., Pastan, I. & Gottesman, M. M. Kinetic evidence suggesting that the multidrug transporter differentially handles influx and efflux of its substrates. Mol. Pharmacol. 45, 763–772 (1994)
Buchler, M. et al. cDNA cloning of the hepatocyte canalicular isoform of the multidrug resistance protein, cMRP, reveals a novel conjugate export pump deficient in the hyperbilirubinemic mutant rats. J. Biol. Chem. 271, 15091–15098 (1996)
Ahmed, M. et al. Two highly similar multidrug transporters of Bacillus subtilis whose expression is differentially regulated. J. Bacteriol. 177, 3904–3910 (1995)
Lewinson, O. & Bibi, E. Evidence for simultaneous binding of dissimilar substrates by the Escherichia coli multidrug transporter MdfA. Biochemistry 40, 12612–12618 (2001)
Loe, D. W., Deeley, R. G. & Cole, S. P. C. Characterisation of vincristine transport by the Mr 190,000 multidrug resistance protein (MRP): evidence for cotransport with reduced glutathione. Cancer Res. 58, 5130–5136 (1998)
Rius, M., Mies, A. J., Hummel-Eisenbeiss, J., Jedlitschky, G. & Keppler, D. Cotransport of reduced glutathione with bile salts by MRP4 (ABCC4) localized to the basolateral hepatocyte membrane. Hepatology 38, 374–384 (2003)
Acknowledgements
This review is dedicated to Alex Neyfakh (1959–2006) who contributed much to our understanding of the multispecificity of drug binding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at www.nature.com/reprints. The author declares no competing financial interests.
Rights and permissions
About this article
Cite this article
Higgins, C. Multiple molecular mechanisms for multidrug resistance transporters. Nature 446, 749–757 (2007). https://doi.org/10.1038/nature05630
Issue Date:
DOI: https://doi.org/10.1038/nature05630
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.