Abstract
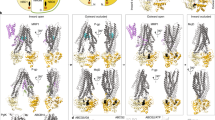
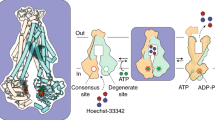
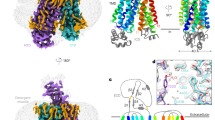
ATP-binding cassette (ABC) transporter proteins carry diverse substrates across cell membranes1. Whereas clinically relevant ABC exporters are implicated in various diseases or cause multidrug resistance of cancer cells2,3, bacterial ABC importers are essential for the uptake of nutrients4, including rare elements such as molybdenum. A detailed understanding of their mechanisms requires direct visualization at high resolution and in distinct conformations. Our recent structure of the multidrug ABC exporter Sav1866 has revealed an outward-facing conformation of the transmembrane domains coupled to a closed conformation of the nucleotide-binding domains, reflecting the ATP-bound state5. Here we present the 3.1 Å crystal structure of a putative molybdate transporter (ModB2C2) from Archaeoglobus fulgidus in complex with its binding protein (ModA). Twelve transmembrane helices of the ModB subunits provide an inward-facing conformation, with a closed gate near the external membrane boundary. The ATP-hydrolysing ModC subunits reveal a nucleotide-free, open conformation, whereas the attached binding protein aligns the substrate-binding cleft with the entrance to the presumed translocation pathway. Structural comparison of ModB2C2A with Sav1866 suggests a common alternating access and release mechanism, with binding of ATP promoting an outward-facing conformation and dissociation of the hydrolysis products promoting an inward-facing conformation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Holland, I. B., Cole, S. P. C., Kuchler, K. & Higgins, C. F. ABC Proteins: From Bacteria to Man (Academic, London, 2003)
Gottesman, M. M. & Ambudkar, S. V. Overview: ABC transporters and human disease. J. Bioenerg. Biomembr. 33, 453–458 (2001)
Gadsby, D. C., Vergani, P. & Csanady, L. The ABC protein turned chloride channel whose failure causes cystic fibrosis. Nature 440, 477–483 (2006)
Nikaido, H. & Hall, J. A. Overview of bacterial ABC transporters. Methods Enzymol. 292, 3–20 (1998)
Dawson, R. J. P. & Locher, K. P. Structure of a bacterial multidrug ABC transporter. Nature 443, 180–185 (2006)
Schneider, E. & Hunke, S. ATP-binding-cassette (ABC) transport systems: Functional and structural aspects of the ATP-hydrolyzing subunits/domains. FEMS Microbiol. Rev. 22, 1–20 (1998)
Rech, S., Deppenmeier, U. & Gunsalus, R. P. Regulation of the molybdate transport operon, modABCD, of Escherichia coli in response to molybdate availability. J. Bacteriol. 177, 1023–1029 (1995)
Mouncey, N. J., Mitchenall, L. A. & Pau, R. N. Mutational analysis of genes of the mod locus involved in molybdenum transport, homeostasis, and processing in Azotobacter vinelandii. J. Bacteriol. 177, 5294–5302 (1995)
Self, W. T., Grunden, A. M., Hasona, A. & Shanmugam, K. T. Molybdate transport. Res. Microbiol. 152, 311–321 (2001)
Chen, J., Sharma, S., Quiocho, F. A. & Davidson, A. L. Trapping the transition state of an ATP-binding cassette transporter: Evidence for a concerted mechanism of maltose transport. Proc. Natl Acad. Sci. USA 98, 1525–1530 (2001)
Locher, K. P., Lee, A. T. & Rees, D. C. The E. coli BtuCD structure: a framework for ABC transporter architecture and mechanism. Science 296, 1091–1098 (2002)
Pinkett, H. W., Lee, A. T., Lum, P., Locher, K. P. & Rees, D. C. An inward-facing conformation of a putative metal-chelate-type ABC transporter. Science 315, 373–377 (2007)
Quiocho, F. A. & Ledvina, P. S. Atomic structure and specificity of bacterial periplasmic receptors for active transport and chemotaxis: variation of common themes. Mol. Microbiol. 20, 17–25 (1996)
Hu, Y., Rech, S., Gunsalus, R. P. & Rees, D. C. Crystal structure of the molybdate binding protein ModA. Nature Struct. Biol. 4, 703–707 (1997)
Lawson, D. M., Williams, C. E., Mitchenall, L. A. & Pau, R. N. Ligand size is a major determinant of specificity in periplasmic oxyanion-binding proteins: the 1.2 A resolution crystal structure of Azotobacter vinelandii ModA. Structure 6, 1529–1539 (1998)
Liu, C. E., Liu, P. G., Wolf, A., Lin, E. & Ames, G. F. Both lobes of the soluble receptor of the periplasmic histidine permease, an ABC transporter (traffic ATPase), interact with the membrane-bound complex. Effect of different ligands and consequences for the mechanism of action. J. Biol. Chem. 274, 739–747 (1999)
Prossnitz, E. Determination of a region of the HisJ binding protein involved in the recognition of the membrane complex of the histidine transport system of Salmonella typhimurium. J. Biol. Chem. 266, 9673–9677 (1991)
Sebulsky, M. T., Shilton, B. H., Speziali, C. D. & Heinrichs, D. E. The role of FhuD2 in iron(III)-hydroxamate transport in Staphylococcus aureus. Demonstration that FhuD2 binds iron(III)-hydroxamates but with minimal conformational change and implication of mutations on transport. J. Biol. Chem. 278, 49890–49900 (2003)
Jones, P. M. & George, A. M. The ABC transporter structure and mechanism: perspectives on recent research. Cell. Mol. Life Sci. 61, 682–699 (2004)
Higgins, C. F. & Linton, K. J. The ATP switch model for ABC transporters. Nature Struct. Mol. Biol. 11, 918–926 (2004)
Hopfner, K. P. et al. Structural biology of Rad50 ATPase: ATP-driven conformational control in DNA double-strand break repair and the ABC-ATPase superfamily. Cell 101, 789–800 (2000)
Smith, P. C. et al. ATP binding to the motor domain from an ABC transporter drives formation of a nucleotide sandwich dimer. Mol. Cell 10, 139–149 (2002)
Chen, J., Lu, G., Lin, J., Davidson, A. L. & Quiocho, F. A. A tweezers-like motion of the ATP-binding cassette dimer in an ABC transport cycle. Mol. Cell 12, 651–661 (2003)
Mannering, D. E., Sharma, S. & Davidson, A. L. Demonstration of conformational changes associated with activation of the maltose transport complex. J. Biol. Chem. 276, 12362–12368 (2001)
Mourez, M., Hofnung, N. & Dassa, E. Subunit interactions in ABC transporters: a conserved sequence in hydrophobic membrane proteins of periplasmic permeases defines an important site of interaction with the ATPase subunits. EMBO J. 16, 3066–3077 (1997)
Loo, T. W. & Clarke, D. M. Recent progress in understanding the mechanism of P-glycoprotein-mediated drug efflux. J. Membr. Biol. 206, 173–185 (2005)
Davidson, A. L. Mechanism of coupling of transport to hydrolysis in bacterial ATP-binding cassette transporters. J. Bacteriol. 184, 1225–1233 (2002)
Sauna, Z. E. & Ambudkar, S. V. Evidence for a requirement for ATP hydrolysis at two distinct steps during a single turnover of the catalytic cycle of human P-glycoprotein. Proc. Natl Acad. Sci. USA 97, 2515–2520 (2000)
Patzlaff, J. S., van der Heide, T. & Poolman, B. The ATP/Substrate stoichiometry of the ATP-binding cassette (ABC) transporter OpuA. J. Biol. Chem. 278, 29546–29551 (2003)
Janas, E. et al. The ATP hydrolysis cycle of the nucleotide-binding domain of the mitochondrial ATP-binding cassette transporter Mdl1p. J. Biol. Chem. 278, 26862–26869 (2003)
Acknowledgements
We thank C. Schulze-Briese, E. Pohl and T. Tomizaki for assistance with synchrotron data collection, B. Blattmann for assistance with initial crystallization screening and J. P. Rosenbusch for discussions. This work was supported by the Roche Research Fund, the National Center for Competence in Research (NCCR) Structural Biology Zurich, and the Swiss National Science Foundation.
Coordinates and structure factors for ModA with bound MoO4, ModA with bound WO4, and for the ModB2C2A complex have been deposited in the Protein Data Bank with accession codes 2ONR, 2ONS, and 2ONK, respectively.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Coordinates and structure factors for ModA with bound MoO4, ModA with bound WO4, and for the ModB2C2A complex have been deposited in the Protein Data Bank with accession codes 2ONR, 2ONS, and 2ONK, respectively. Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Methods, Supplementary Tables S1-S2, Supplementary Figures S1-S5 and additional references. (PDF 1040 kb)
Rights and permissions
About this article
Cite this article
Hollenstein, K., Frei, D. & Locher, K. Structure of an ABC transporter in complex with its binding protein. Nature 446, 213–216 (2007). https://doi.org/10.1038/nature05626
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature05626
This article is cited by
-
Solution NMR chemical shift assignment of apo and molybdate-bound ModA at two pHs
Biomolecular NMR Assignments (2024)
-
Integrative physiological and metabolic traits reveal the mechanisms of chamomile flowers in response to nicotine stress
Chemical and Biological Technologies in Agriculture (2023)
-
tRNA-derived fragments from wheat are potentially involved in susceptibility to Fusarium head blight
BMC Plant Biology (2022)
-
Genome-wide identification of ATP binding cassette (ABC) transporter and heavy metal associated (HMA) gene families in flax (Linum usitatissimum L.)
BMC Genomics (2020)
-
mRNA profile provides novel insights into stress adaptation in mud crab megalopa, Scylla paramamosain after salinity stress
BMC Genomics (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.