Abstract
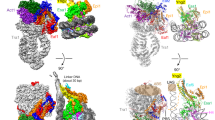
CIA (CCG1-interacting factor A)/ASF1, which is the most conserved histone chaperone among the eukaryotes, was genetically identified as a factor for an anti-silencing function (Asf1)1 by yeast genetic screening. Shortly after that, the CIA–histone-H3–H4 complex was isolated from Drosophila as a histone chaperone CAF-1 stimulator2. Human CIA-I/II (ASF1a/b) was identified as a histone chaperone that interacts with the bromodomain—an acetylated-histone-recognizing domain—of CCG1, in the general transcription initiation factor TFIID3,4,5. Intensive studies have revealed that CIA/ASF1 mediates nucleosome assembly by forming a complex with another histone chaperone in human cells6 and yeast7, and is involved in DNA replication1,2, transcription4,8,9,10, DNA repair1,2,11,12 and silencing/anti-silencing1,2,8,13,14,15 in yeast. CIA/ASF1 was shown as a major storage chaperone for soluble histones in proliferating human cells6,16. Despite all these biochemical and biological functional analyses, the structure–function relationship of the nucleosome assembly/disassembly activity of CIA/ASF1 has remained elusive. Here we report the crystal structure, at 2.7 Å resolution, of CIA-I in complex with histones H3 and H4. The structure shows the histone H3–H4 dimer's mutually exclusive interactions with another histone H3–H4 dimer and CIA-I. The carboxy-terminal β-strand of histone H4 changes its partner from the β-strand in histone H2A to that of CIA-I through large conformational change. In vitro functional analysis demonstrated that CIA-I has a histone H3–H4 tetramer-disrupting activity. Mutants with weak histone H3–H4 dimer binding activity showed critical functional effects on cellular processes related to transcription. The histone H3–H4 tetramer-disrupting activity of CIA/ASF1 and the crystal structure of the CIA/ASF1–histone-H3–H4 dimer complex should give insights into mechanisms of both nucleosome assembly/disassembly and nucleosome semi-conservative replication.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Le, S., Davis, C., Konopka, J. B. & Sternglanz, R. Two new S-phase-specific genes from Saccharomyces cerevisiae. Yeast 13, 1029–1042 (1997)
Tyler, J. K. et al. The RCAF complex mediates chromatin assembly during DNA replication and repair. Nature 402, 555–560 (1999)
Munakata, T., Adachi, N., Yokoyama, N., Kuzuhara, T. & Horikoshi, M. A human homologue of yeast anti-silencing factor has histone chaperone activity. Genes Cells 5, 221–233 (2000)
Chimura, T., Kuzuhara, T. & Horikoshi, M. Identification and characterization of CIA/ASF1 as an interactor of bromodomains associated with TFIID. Proc. Natl Acad. Sci. USA 99, 9334–9339 (2002)
Umehara, T. & Horikoshi, M. Transcription initiation factor IID-interactive histone chaperone CIA-II implicated in mammalian spermatogenesis. J. Biol. Chem. 278, 35660–35667 (2003)
Tagami, H., Ray-Gallet, D., Almouzni, G. & Nakatani, Y. Histone H3.1 and H3.3 complexes mediate nucleosome assembly pathways dependent or independent of DNA synthesis. Cell 116, 51–61 (2004)
Green, E. M. et al. Replication-independent histone deposition by the HIR complex and Asf1. Curr. Biol. 15, 2044–2049 (2005)
Sharp, J. A., Fouts, E. T., Krawitz, D. C. & Kaufman, P. D. Yeast histone deposition protein Asf1p requires Hir proteins and PCNA for heterochromatic silencing. Curr. Biol. 11, 463–473 (2001)
Adkins, M. W., Howar, S. R. & Tyler, J. K. Chromatin disassembly mediated by the histone chaperone Asf1 is essential for transcriptional activation of the yeast PHO5 and PHO8 genes. Mol. Cell 14, 657–666 (2004)
Schwabish, M. A. & Struhl, K. Asf1 mediates histone eviction and deposition during elongation by RNA polymerase II. Mol. Cell 22, 415–422 (2006)
Emili, A., Schieltz, D. M., Yates, J. R. & Hartwell, L. H. Dynamic interaction of DNA damage checkpoint protein Rad53 with chromatin assembly factor Asf1. Mol. Cell 7, 13–20 (2001)
Hu, F., Alcasabas, A. & Elledge, A. J. Asf1 links Rad53 to control of chromatin assembly. Genes Dev. 15, 1061–1066 (2001)
Osada, S. et al. The yeast SAS (something about silencing) protein complex contains a MYST-type putative acetyltransferase and functions with chromatin assembly factor ASF1. Genes Dev. 15, 3155–3168 (2001)
Meijsing, S. H. & Ehrenhofer-Murray, A. E. The silencing complex SAS-I links histone acetylation to the assembly of repressed chromatin by CAF-I and Asf1 in Saccharomyces cerevisiae. Genes Dev. 15, 3169–3182 (2001)
Umehara, T., Chimura, T., Ichikawa, N. & Horikoshi, M. Polyanionic stretch-deleted histone chaperone cia1/Asf1p is functional both in vivo and in vitro. Genes Cells 7, 59–73 (2002)
Groth, A. et al. Human Asf1 regulates the flow of S phase histones during replicational stress. Mol. Cell 17, 301–311 (2005)
Sillje, H. H. & Nigg, E. A. Identification of human Asf1 chromatin assembly factors as substrates of Tousled-like kinases. Curr. Biol. 11, 1068–1073 (2001)
English, C. M., Maluf, N. K., Tripet, B., Churchill, M. E. & Tyler, J. K. ASF1 binds to a heterodimer of histones H3 and H4: a two-step mechanism for the assembly of the H3–H4 heterotetramer on DNA. Biochemistry 44, 13673–13682 (2005)
Strahl, B. D. & Allis, C. D. The language of covalent histone modifications. Nature 403, 41–45 (2000)
Cosgrove, M. S., Boeke, J. D. & Wolberger, C. Regulated nucleosome mobility and the histone code. Nature Struct. Mol. Biol. 11, 1037–1043 (2004)
Luger, K., Mäder, A. W., Richmond, R. K., Sargent, D. F. & Richmond, T. J. Crystal structure of the nucleosome core particle at 2.8 Å resolution. Nature 389, 251–260 (1997)
Mousson, F. et al. Structural basis for the interaction of Asf1 with histone H3 and its functional implications. Proc. Natl Acad. Sci. USA 102, 5975–5980 (2005)
Silverman, S. J. & Fink, G. R. Effects of Ty insertions on HIS4 transcription in Saccharomyces cerevisiae. Mol. Cell. Biol. 4, 1246–1251 (1984)
Yamaguchi, Y., Narita, T., Inukai, N., Wada, T. & Handa, H. SPT genes: key players in the regulation of transcription, chromatin structure and other cellular processes. J. Biochem. 129, 185–191 (2001)
Matsubara, K., Sano, N., Umehara, T. & Horikoshi, M. Global analysis of functional surfaces of core histones with comprehensive point mutants. Genes Cells 12, 13–33 (2007)
Collaborative computational project number 4. CCP4 suite: Programs for protein crystallography by collaborative computational project number 4. Acta Crystallogr. D. 50, 760–763 (1994)
Abramoff, M. D., Magelhaes, P. J. & Ram, S. J. Image processing with ImageJ. Biophotonics International 11, 36–42 (2004)
Tang, Y. et al. Structure of a human ASF1–HIRA complex and insights into specificity of histone chaperone complex assembly. Nature Struct. Mol. Biol. 13, 921–929 (2006)
DeLano, W. L. The PyMOL Molecular Graphics System 〈http://www.pymol.org〉 (2006)
English, C. M., Adkins, M. W., Carson, J. J., Churchill, M. E. & Tyler, J. K. Structure basis of the histone chaperone activity of Asf1. Cell 127, 495–508 (2006)
Acknowledgements
We thank T. K. Kundu for the expression vector of Xenopus histones; F. Winston for yeast strains; K. Matsubara, Y. Ikejiri, S. Okano and S. Yoshihara for the construction of the histone mutants; and K. Hasegawa for critical reading of the manuscript. This study was supported in part by the New Energy and Industrial Technology Development Organization (NEDO) of Japan, the Exploratory Research for Advanced Technology (ERATO) of the Japan Science and Technology Agency (JST), and Ministry of Education, Culture, Sports, Science and Technology of Japan.
Author Contributions R.N. and M.E. contributed equally to this work. R.N. and Y.A. performed crystallographic and biochemical works. M.E. and N.S. performed biochemical works and yeast genetics. T.S. performed crystallographic work. M.H. and T.S. contributed to the idea, strategy, project management and writing of the manuscript. All authors discussed the results and commented on the manuscript.
The atomic coordinates have been deposited in the Protein Data Bank (PDB code, 2IO5).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The atomic coordinates have been deposited in the Protein Data Bank (PDB code, 2IO5). Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-9 with Legends, Supplementary Methods, Supplementary Tables 1-3, and additional references. (PDF 2148 kb)
Rights and permissions
About this article
Cite this article
Natsume, R., Eitoku, M., Akai, Y. et al. Structure and function of the histone chaperone CIA/ASF1 complexed with histones H3 and H4. Nature 446, 338–341 (2007). https://doi.org/10.1038/nature05613
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature05613
This article is cited by
-
The patterns and participants of parental histone recycling during DNA replication in Saccharomyces cerevisiae
Science China Life Sciences (2023)
-
miR-24-3p Regulates Epithelial–Mesenchymal Transition and the Malignant Phenotype of Pancreatic Adenocarcinoma by Regulating ASF1B Expression
Biochemical Genetics (2023)
-
Histone 3.3-related chromatinopathy: missense variants throughout H3-3A and H3-3B cause a range of functional consequences across species
Human Genetics (2023)
-
Histone chaperone ASF1 mediates H3.3-H4 deposition in Arabidopsis
Nature Communications (2022)
-
Distinct role of histone chaperone Asf1a and Asf1b during fertilization and pre-implantation embryonic development in mice
Epigenetics & Chromatin (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.