Abstract
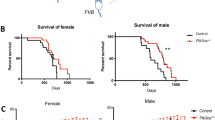
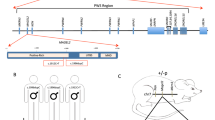
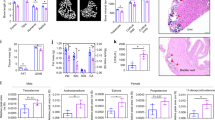
Glycogen synthase kinase-3β (GSK-3β) has integral roles in a variety of biological processes, including development, diabetes, and the progression of Alzheimer’s disease1,2,3,4. As such, a thorough understanding of GSK-3β function will have a broad impact on human biology and therapeutics. Because GSK-3β interacts with many different pathways, its specific developmental roles remain unclear5. We have discovered a genetic requirement for GSK-3β in midline development. Homozygous null mice display cleft palate, incomplete fusion of the ribs at the midline and bifid sternum as well as delayed sternal ossification. Using a chemically regulated allele of GSK-3β (ref. 6), we have defined requirements for GSK-3β activity during discrete temporal windows in palatogenesis and skeletogenesis. The rapamycin-dependent allele of GSK-3β produces GSK-3β fused to a tag, FRB* (FKBP/rapamycin binding), resulting in a rapidly destabilized chimaeric protein. In the absence of drug, GSK-3βFRB*/FRB* mutants appear phenotypically identical to GSK-3β-/- mutants. In the presence of drug, GSK-3βFRB* is rapidly stabilized, restoring protein levels and activity6. Using this system, mutant phenotypes were rescued by restoring endogenous GSK-3β activity during two distinct periods in gestation. This technology provides a powerful tool for defining windows of protein function during development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Cohen, P. & Frame, S. The renaissance of GSK3. Nature Rev. Mol. Cell Biol. 2, 769–776 (2001)
Frame, S. & Cohen, P. GSK3 takes centre stage more than 20 years after its discovery. Biochem. J. 359, 1–16 (2001)
Doble, B. W. & Woodgett, J. R. GSK-3: tricks of the trade for a multi-tasking kinase. J. Cell Sci. 116, 1175–1186 (2003)
Jope, R. S. & Johnson, G. V. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem. Sci. 29, 95–102 (2004)
Hoeflich, K. P. et al. Requirement for glycogen synthase kinase-3β in cell survival and NF-κB activation. Nature 406, 86–90 (2000)
Stankunas, K. et al. Conditional protein alleles using knockin mice and a chemical inducer of dimerization. Mol. Cell 12, 1615–1624 (2003)
Murray, J. C. & Schutte, B. C. Cleft palate: players, pathways, and pursuits. J. Clin. Invest. 113, 1676–1678 (2004)
Chen, J. K., Taipale, J., Cooper, M. K. & Beachy, P. A. Inhibition of Hedgehog signaling by direct binding of cyclopamine to Smoothened. Genes Dev. 16, 2743–2748 (2002)
Graef, I. A., Chen, F., Chen, L., Kuo, A. & Crabtree, G. R. Signals transduced by Ca2+/calcineurin and NFATc3/c4 pattern the developing vasculature. Cell 105, 863–875 (2001)
Graef, I. A. et al. Neurotrophins and netrins require calcineurin/NFAT signaling to stimulate outgrowth of embryonic axons. Cell 113, 657–670 (2003)
Peterson, R. T. et al. Chemical suppression of a genetic mutation in a zebrafish model of aortic coarctation. Nature Biotechnol. 22, 595–599 (2004)
Peterson, R. T., Link, B. A., Dowling, J. E. & Schreiber, S. L. Small molecule developmental screens reveal the logic and timing of vertebrate development. Proc. Natl Acad. Sci. USA 97, 12965–12969 (2000)
Liu, J. et al. A small-molecule agonist of the Wnt signaling pathway. Angew. Chem. Int. Ed. 44, 1987–1990 (2005)
Sharpe, P. M. & Ferguson, M. W. Mesenchymal influences on epithelial differentiation in developing systems. J. Cell Sci. 10 , (suppl.)195–230 (1988)
Karsenty, G. & Wagner, E. F. Reaching a genetic and molecular understanding of skeletal development. Dev. Cell 2, 389–406 (2002)
Krishnan, V., Bryant, H. U. & Macdougald, O. A. Regulation of bone mass by Wnt signaling. J. Clin. Invest. 116, 1202–1209 (2006)
Chen, J. M. Studies on the morphogenesis of the mouse sternum. I. Normal embryonic development. J. Anat. 86, 373–386 (1952)
Ito, Y. et al. Conditional inactivation of Tgfbr2 in cranial neural crest causes cleft palate and calvaria defects. Development 130, 5269–5280 (2003)
Hentges, K. E. et al. FRAP/mTOR is required for proliferation and patterning during embryonic development in the mouse. Proc. Natl Acad. Sci. USA 98, 13796–13801 (2001)
Flake, A. W., Villa, R. L., Adzick, N. S. & Harrison, M. R. Transamniotic fetal feeding. II. A model of intrauterine growth retardation using the relationship of “natural runting” to uterine position. J. Pediatr. Surg. 22, 816–819 (1987)
Acknowledgements
We thank M. S. Dionne for critical reading of the manuscript; M. S. Dionne, M. M. Winslow, J. E. Gestwicki, J. H. Bayle, S. C. Kao and members of the Longaker and Crabtree laboratories for invaluable discussions; and J. Woodgett for the gift of GSK-3β knockout mice. K.J.L and M.T.L. are supported by the NIH, M.T.L. is also supported by the Oak Foundation and J.R.A. is a fellow of the Berry Foundation.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Methods and Supplementary Figures S1-S2 with legends. (PDF 308 kb)
Rights and permissions
About this article
Cite this article
Liu, K., Arron, J., Stankunas, K. et al. Chemical rescue of cleft palate and midline defects in conditional GSK-3β mice. Nature 446, 79–82 (2007). https://doi.org/10.1038/nature05557
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature05557
This article is cited by
-
Multimodal spatiotemporal transcriptomic resolution of embryonic palate osteogenesis
Nature Communications (2023)
-
Glycogen synthase kinase 3 controls migration of the neural crest lineage in mouse and Xenopus
Nature Communications (2018)
-
Abrogation of TGF-beta signalling in TAGLN expressing cells recapitulates Pentalogy of Cantrell in the mouse
Scientific Reports (2018)
-
EGFR controls bone development by negatively regulating mTOR-signaling during osteoblast differentiation
Cell Death & Differentiation (2018)
-
Glycogen Synthase Kinase-3β Inhibition Links Mitochondrial Dysfunction, Extracellular Matrix Remodelling and Terminal Differentiation in Chondrocytes
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.