Abstract
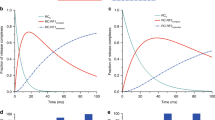
The contribution of co-translational chaperone functions to protein folding is poorly understood. Ribosome-associated trigger factor (TF) is the first molecular chaperone encountered by nascent polypeptides in bacteria. Here we show, using fluorescence spectroscopy to monitor TF function and structural rearrangements in real time, that TF interacts with ribosomes and translating polypeptides in a dynamic reaction cycle. Ribosome binding stabilizes TF in an open, activated conformation. Activated TF departs from the ribosome after a mean residence time of ∼10 s, but may remain associated with the elongating nascent chain for up to 35 s, allowing entry of a new TF molecule at the ribosome docking site. The duration of nascent-chain interaction correlates with the occurrence of hydrophobic motifs in translating polypeptides, reflecting a high aggregation propensity. These findings can explain how TF prevents misfolding events during translation and may provide a paradigm for the regulation of nucleotide-independent chaperones.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Frydman, J. Folding of newly translated proteins in vivo: the role of molecular chaperones. Annu. Rev. Biochem. 70, 603–647 (2001)
Young, J. C., Agashe, V. R., Siegers, K. & Hartl, F. U. Pathways of chaperone-mediated protein folding in the cytosol. Nature Rev. Mol. Cell Biol. 5, 781–791 (2004)
Deuerling, E. & Bukau, B. Chaperone-assisted folding of newly synthesized proteins in the cytosol. Crit. Rev. Biochem. Mol. Biol. 39, 261–277 (2004)
Kramer, G. et al. L23 protein functions as a chaperone docking site on the ribosome. Nature 419, 171–174 (2002)
Schlunzen, F. et al. The binding mode of the trigger factor on the ribosome: implications for protein folding and SRP interaction. Structure (Camb.) 13, 1685–1694 (2005)
Baram, D. et al. Structure of trigger factor binding domain in biologically homologous complex with eubacterial ribosome reveals its chaperone action. Proc. Natl Acad. Sci. USA 102, 12017–12022 (2005)
Hesterkamp, T., Hauser, S., Lutcke, H. & Bukau, B. Escherichia coli trigger factor is a prolyl isomerase that associates with nascent polypeptide chains. Proc. Natl Acad. Sci. USA 93, 4437–4441 (1996)
Valent, Q. A. et al. Early events in preprotein recognition in E. coli: interaction of SRP and trigger factor with nascent polypeptides. EMBO J. 14, 5494–5505 (1995)
Hartl, F. U. & Hayer-Hartl, M. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science 295, 1852–1858 (2002)
Agashe, V. R. et al. Function of trigger factor and DnaK in multidomain protein folding: increase in yield at the expense of folding speed. Cell 117, 199–209 (2004)
Chang, H. C., Kaiser, C. M., Hartl, F. U. & Barral, J. M. De novo folding of GFP fusion proteins: high efficiency in eukaryotes but not in bacteria. J. Mol. Biol. 353, 397–409 (2005)
Bremer, H. & Dennis, P. P. in Escherichia coli and Salmonella: Cellular and Molecular Biology (ed. Neidhart, F. C.) 1553–1569 (ASM Press, Washington DC, 1996)
Lill, R., Crooke, E., Guthrie, B. & Wickner, W. The “trigger factor cycle” includes ribosomes, presecretory proteins, and the plasma membrane. Cell 54, 1013–1018 (1988)
Maier, R., Eckert, B., Scholz, C., Lilie, H. & Schmid, F. X. Interaction of trigger factor with the ribosome. J. Mol. Biol. 326, 585–592 (2003)
Ferbitz, L. et al. Trigger factor in complex with the ribosome forms a molecular cradle for nascent proteins. Nature 431, 590–596 (2004)
Ludlam, A. V., Moore, B. A. & Xu, Z. The crystal structure of ribosomal chaperone trigger factor from Vibrio cholerae. Proc. Natl Acad. Sci. USA 101, 13436–13441 (2004)
Genevaux, P. et al. In vivo analysis of the overlapping functions of DnaK and trigger factor. EMBO Rep. 5, 195–200 (2004)
Kramer, G. et al. Functional dissection of Escherichia coli trigger factor: unraveling the function of individual domains. J. Bacteriol. 186, 3777–3784 (2004)
Kramer, G. et al. Trigger factor peptidyl-prolyl cis/trans isomerase activity is not essential for the folding of cytosolic proteins in Escherichia coli.. J. Biol. Chem. 279, 14165–14170 (2004)
Stryer, L. Fluorescence spectroscopy of proteins. Science 162, 526–533 (1968)
Woolhead, C. A., McCormick, P. J. & Johnson, A. E. Nascent membrane and secretory proteins differ in FRET-detected folding far inside the ribosome and in their exposure to ribosomal proteins. Cell 116, 725–736 (2004)
Schroder, G. F. & Grubmuller, H. FRETsg: biomolecular structure model building from multiple FRET experiments. Comp. Phys. Comm. 158, 150–157 (2004)
Patzelt, H. et al. Three-state equilibrium of Escherichia coli trigger factor. Biol. Chem. 383, 1611–1619 (2002)
Shimizu, Y. et al. Cell-free translation reconstituted with purified components. Nature Biotechnol. 19, 751–755 (2001)
Patzelt, H. et al. Binding specificity of Escherichia coli trigger factor. Proc. Natl Acad. Sci. USA 98, 14244–14249 (2001)
Chin, J. W., Martin, A. B., King, D. S., Wang, L. & Schultz, P. G. Addition of a photocrosslinking amino acid to the genetic code of Escherichia coli. Proc. Natl Acad. Sci. USA 99, 11020–11024 (2002)
Michalski, C. J., Sells, B. H. & Morrison, M. Molecular morphology of ribosomes. Localization of ribosomal proteins in 50-S subunits. Eur. J. Biochem. 33, 481–485 (1973)
Rudiger, S., Germeroth, L., Schneider-Mergener, J. & Bukau, B. Substrate specificity of the DnaK chaperone determined by screening cellulose-bound peptide libraries. EMBO J. 16, 1501–1507 (1997)
Roseman, M. A. Hydrophilicity of polar amino acid side-chains is markedly reduced by flanking peptide bonds. J. Mol. Biol. 200, 513–522 (1988)
Creighton, T. E. Proteins: Structures and Molecular Properties (W. H. Freeman and Co., New York, 1984)
Conti, E., Franks, N. P. & Brick, P. Crystal structure of firefly luciferase throws light on a superfamily of adenylate-forming enzymes. Structure 4, 287–298 (1996)
Improta, S., Politou, A. S. & Pastore, A. Immunoglobulin-like modules from titin I-band: extensible components of muscle elasticity. Structure 4, 323–337 (1996)
Marszalek, P. E. et al. Mechanical unfolding intermediates in titin modules. Nature 402, 100–103 (1999)
Ban, N., Nissen, P., Hansen, J., Moore, P. B. & Steitz, T. A. The complete atomic structure of the large ribosomal subunit at 2.4 Å resolution. Science 289, 905–920 (2000)
Harms, J. et al. High resolution structure of the large ribosomal subunit from a mesophilic eubacterium. Cell 107, 679–688 (2001)
Ullers, R. S. et al. Interplay of signal recognition particle and trigger factor at L23 near the nascent chain exit site on the Escherichia coli ribosome. J. Cell Biol. 161, 679–684 (2003)
Beck, K., Wu, L. F., Brunner, J. & Muller, M. Discrimination between SRP- and SecA/SecB-dependent substrates involves selective recognition of nascent chains by SRP and trigger factor. EMBO J. 19, 134–143 (2000)
Eisner, G., Moser, M., Schafer, U., Beck, K. & Muller, M. Alternate recruitment of signal recognition particle and trigger factor to the signal sequence of a growing nascent polypeptide. J. Biol. Chem. 281, 7172–7179 (2006)
Buskiewicz, I. et al. Trigger factor binds to ribosome-signal-recognition particle (SRP) complexes and is excluded by binding of the SRP receptor. Proc. Natl Acad. Sci. USA 101, 7902–7906 (2004)
Raine, A., Ivanova, N., Wikberg, J. E. & Ehrenberg, M. Simultaneous binding of trigger factor and signal recognition particle to the E. coli ribosome. Biochimie 86, 495–500 (2004)
Ullers, R. S. et al. Sequence-specific interactions of nascent Escherichia coli polypeptides with trigger factor and signal recognition particle. J. Biol. Chem. 281, 13999–14005 (2006)
Chiti, F., Stefani, M., Taddei, N., Ramponi, G. & Dobson, C. M. Rationalization of the effects of mutations on peptide and protein aggregation rates. Nature 424, 805–808 (2003)
Spedding, G. in Ribosomes and Protein Synthesis: A Practical Approach (ed. Spedding, G.) 1–29 (Oxford Univ. Press, Oxford, 1990)
Kerner, M. J. et al. Proteome-wide analysis of chaperonin-dependent protein folding in Escherichia coli.. Cell 122, 209–220 (2005)
Acknowledgements
We thank A. Johnson for advice regarding assessment of fluorophore mobility, A. Bracher for help in designing the ΔPPIase construct and advice during building of the TF dimer model, M. Kerner for his help with bioinformatics analyses, R. Boteva and K. Chakroborty for discussion, P. Genevaux and C. Georgopoulos for the GP 367 E. coli strain and P. Schultz for reagents for in vivo crosslinking. J.M.B. was supported by a fellowship from the International Human Frontier Science Program Organization. Support by the Ernst Jung Foundation and the European Union is also acknowledged. Author Contributions F.U.H. was the project leader. C.M.K. and J.M.B. were responsible for experimental and project design. C.M.K. performed most of the experiments. V.R.A. made conceptual contributions and H.-C.C., V.R.A., S.K.L., S.A.E., M.H.-H. and J.M.B. performed some of the experiments. F.U.H. and J.M.B. wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1–6, Supplementary Methods and Supplementary Notes. (PDF 512 kb)
Rights and permissions
About this article
Cite this article
Kaiser, C., Chang, HC., Agashe, V. et al. Real-time observation of trigger factor function on translating ribosomes. Nature 444, 455–460 (2006). https://doi.org/10.1038/nature05225
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature05225
This article is cited by
-
Functional cooperativity between the trigger factor chaperone and the ClpXP proteolytic complex
Nature Communications (2021)
-
A Review: Molecular Chaperone-mediated Folding, Unfolding and Disaggregation of Expressed Recombinant Proteins
Cell Biochemistry and Biophysics (2021)
-
The dynamic dimer structure of the chaperone Trigger Factor
Nature Communications (2017)
-
Protein export through the bacterial Sec pathway
Nature Reviews Microbiology (2017)
-
Structural and molecular comparison of bacterial and eukaryotic trigger factors
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.