Abstract
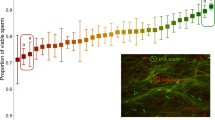
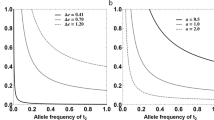
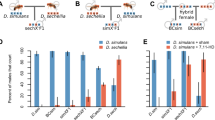
Females often mate with several males before producing offspring1. Field studies of vertebrates suggest, and laboratory experiments on invertebrates confirm, that even when males provide no material benefits, polyandry can enhance offspring survival2,3. This enhancement is widely attributed to genetic benefits that arise whenever paternity is biased towards males that sire more viable offspring1,4,5. Field studies suggest that post-mating sexual selection biases fertilization towards genetically more compatible males6,7 and one controlled experiment has shown that, when females mate with close kin, polyandry reduces the relative number of inbred offspring8. Another potential genetic benefit of polyandry is that it increases offspring survival because males with more competitive ejaculates sire more viable offspring9. Surprisingly, however, there is no unequivocal evidence for this process10. Here, by experimentally assigning mates to females, we show that polyandry greatly increases offspring survival in the Australian marsupial Antechinus stuartii. DNA profiling shows that males that gain high paternity under sperm competition sire offspring that are more viable. This beneficial effect occurs in both the laboratory and the wild. Crucially, there are no confounding non-genetic maternal effects that could arise if polyandry increases female investment in a particular reproductive event10 because A. stuartii is effectively semelparous. Our results therefore show that polyandry improves female lifetime fitness in nature. The threefold increase in offspring survival is not negated by a decline in maternal lifespan and is too large to be offset by an equivalent decline in the reproductive performance of surviving offspring.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Jennions, M. D. & Petrie, M. Why do females mate multiply? A review of the genetic benefits. Biol. Rev. Camb. Phil. Soc. 75, 21–64 (2000)
Madsen, T., Shine, R., Loman, J. & Hakansson, T. Why do female adders copulate so frequently?. Nature 335, 440–441 (1992)
Tregenza, T. & Wedell, N. Benefits of multiple mates in the cricket Gryllus bimaculatus. Evolution Int. J. Org. Evolution 52, 1726–1730 (1998)
Tregenza, T. & Weddell, N. Genetic compatibility, mate choice and patterns of parentage. Mol. Ecol. 9, 1013–1027 (2000)
Zeh, J. A. & Zeh, D. W. Reproductive mode and the genetic benefits of polyandry. Anim. Behav. 61, 1051–1063 (2001)
Olsson, M., Shine, R., Madsen, T., Gullberg, A. & Tegelstrom, H. Sperm selection by females. Nature 383, 585 (1996)
Thuman, K. A. & Griffith, S. C. Genetic similarity and the non-random distribution of paternity in a genetically highly polyandrous shorebird. Anim. Behav. 69, 765–770 (2005)
Tregenza, T. & Wedell, N. Polyandrous females avoid costs of inbreeding. Nature 415, 71–73 (2002)
Yasui, Y. A ‘good sperm’ model can explain the evolution of costly multiple mating by females. Am. Nat. 149, 573–584 (1997)
Simmons, L. W. The evolution of polyandry: sperm competition, sperm selection, and offspring viability. Annu. Rev. Ecol. Evol. Syst. 36, 125–146 (2005)
Tregenza, T., Wedell, N., Hosken, D. J. & Ward, P. I. Maternal effects on offspring depend on female mating pattern and offspring environment in yellow dung flies. Evolution Int. J. Org. Evolution 57, 297–304 (2003)
Sheldon, B. C. Differential allocation: tests, mechanisms and implications. Trends Ecol. Evol. 15, 397–402 (2000)
Kozielska, M., Krzeminska, A. & Radwan, J. Good genes and the maternal effects of polyandry on offspring reproductive success in the bulb mite. Proc. R. Soc. Lond. B 271, 165–170 (2004)
Weigensberg, I., Carriére, Y. & Roff, D. A. Effects of male genetic contribution and paternal investment to egg and hatchling size in the cricket Gryllus firmus. J. Evol. Biol. 11, 135–146 (1998)
Zeh, J. A. & Zeh, D. W. Outbred embryos rescue inbred half-siblings in mixed-paternity broods of live-bearing females. Nature 439, 201–203 (2006)
Evans, J. P., Zane, L., Francescato, S. & Pilastro, A. Directional postcopulatory sexual selection revealed by artificial insemination. Nature 421, 360–363 (2003)
Hosken, D. J., Garner, T. W. J., Tregenza, T., Wedell, N. & Ward, P. I. Superior sperm competitors sire higher-quality young. Proc. R. Soc. Lond. B 2270, 1933–1938 (2003)
Holleley, C. E., Dickman, C. R., Crowther, M. S. & Oldroyd, B. P. Size breeds success: multiple paternity, multivariate selection and male semelparity in a small marsupial, Antechinus stuartii. Mol. Ecol. 15, 3439–3448 (2006)
Selwood, L. A timetable of embryonic development of the dasyurid marsupial Antechinus stuartii (Macleay). Aust. J. Zool. 28, 649–668 (1980)
Fisher, D. O. Population density and presence of the mother are related to natal dispersal decisions in male and female Antechinus stuartii. Aust. J. Zool. 53, 103–110 (2005)
Fisher, D. O. & Cockburn, A. The large male advantage in brown antechinuses: female choice, male dominance and delayed male death. Behav. Ecol. 17, 164–171 (2005)
Kraaijeveld-Smit, F. J. L., Ward, S. J. & Temple-Smith, P. D. Paternity success and the direction of sexual selection in a field population of a semelparous marsupial, Antechinus agilis. Mol. Ecol. 12, 475–484 (2003)
Fisher, D. O., Double, M. C. & Moore, B. D. Number of mates and timing of mating affect offspring growth in the small marsupial, Antechinus agilis. Anim. Behav. 71, 289–297 (2006)
Neff, B. D. & Pitcher, T. E. Genetic quality and sexual selection: an integrated framework for good genes and compatible genes. Mol. Ecol. 14, 19–38 (2005)
Evans, J. P. & Marshall, D. J. Male-by-female interactions influence fertilization success and mediate the benefits of polyandry in the sea urchin Heliocidaris erythrogramma. Evolution Int. J. Org. Evolution 59, 106–112 (2005)
Nicholls, J. A., Double, M. C., Rowell, D. & Magrath, R. D. The evolution of cooperative and pair breeding in thornbills Acanthiza (Aves: Pardalotidae). J. Avian Biol. 31, 165–176 (2000)
Bates, D. & Maechler, M. Matrix: a matrix package for R (R Foundation for Statistical Computing, Vienna, 2006)
R Core Development Team. R: a language and environment for statistical computing (R Foundation for Statistical Computing, Vienna, 2006)
Genstat Committee. Genstat Manual, version 8 (Clarendon, Oxford, UK, 2005)
Pollock, K. H., Nichols, J. D., Brownie, C. & Hines, J. E. Statistical inference for capture-recapture experiments. Wildlife Monogr. 107, 1–97 (1990)
Acknowledgements
We thank P. Backwell, S. Griffith and M. Olsson for discussions and comments; numerous field and lab assistants for their hard work; P. Marsack, S. and R. Berkhout, and O. and C. Carriage for help at Kioloa. This work was supported by an Australian Research Council fellowship (to D.O.F.). Author Contributions D.O.F. designed the main study, carried out the experiments, performed animal husbandry and drafted the manuscript. M.C.D. performed the molecular analysis. S.P.B. performed statistical analyses and contributed to fieldwork. A.C. carried out additional statistical analyses; M.D.J. designed the sperm competition experiment. All authors discussed the results and analysis and contributed to the writing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests.
Supplementary information
Supplementary Methods and Supplementary Discussion
This file contains details of the methods for genotyping, statistical analysis and discussion of the effect of treatment on offspring sex ratio and survival, and offspring growth. This file also contains an elaboration of the combined effects of mating treatment across years, our choice of methods to analyse the number of young attached at birth, and of the effect of ejaculate competitiveness on offspring survival, and an explanation of the evidence concerning relatedness between mates, and of the extraordinary life history and sexual behaviour of male antechinuses. (DOC 43 kb)
Rights and permissions
About this article
Cite this article
Fisher, D., Double, M., Blomberg, S. et al. Post-mating sexual selection increases lifetime fitness of polyandrous females in the wild. Nature 444, 89–92 (2006). https://doi.org/10.1038/nature05206
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature05206
This article is cited by
-
Polyandry and non-random fertilisation maintain long-term genetic diversity in an isolated island population of adders (Vipera berus)
Heredity (2023)
-
Rare polyandry and common monogamy in the firefly squid, Watasenia scintillans
Scientific Reports (2020)
-
Fitness costs of polyandry to female cigarette beetle Lasioderma serricorne
Behavioral Ecology and Sociobiology (2017)
-
Does the degree of endocrine dyscrasia post-reproduction dictate post-reproductive lifespan? Lessons from semelparous and iteroparous species
GeroScience (2017)
-
Individual, social, and sexual niche traits affect copulation success in a polygynandrous mating system
Behavioral Ecology and Sociobiology (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.