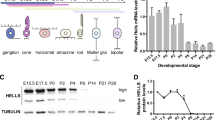
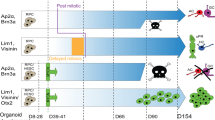
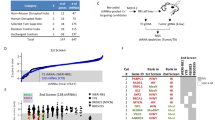
Abstract
Most human tumours have genetic mutations in their Rb and p53 pathways, but retinoblastoma is thought to be an exception. Studies suggest that retinoblastomas, which initiate with mutations in the gene retinoblastoma 1 (RB1), bypass the p53 pathway because they arise from intrinsically death-resistant cells during retinal development. In contrast to this prevailing theory, here we show that the tumour surveillance pathway mediated by Arf, MDM2, MDMX and p53 is activated after loss of RB1 during retinogenesis. RB1-deficient retinoblasts undergo p53-mediated apoptosis and exit the cell cycle. Subsequently, amplification of the MDMX gene and increased expression of MDMX protein are strongly selected for during tumour progression as a mechanism to suppress the p53 response in RB1-deficient retinal cells. Our data provide evidence that the p53 pathway is inactivated in retinoblastoma and that this cancer does not originate from intrinsically death-resistant cells as previously thought. In addition, they support the idea that MDMX is a specific chemotherapeutic target for treating retinoblastoma.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hahn, W. C. & Weinberg, R. A. Modelling the molecular circuitry of cancer. Nature Rev. Cancer 2, 331–341 (2002)
Vogelstein, B. & Kinzler, K. W. Cancer genes and the pathways they control. Nature Med. 10, 789–799 (2004)
Sherr, C. J. & McCormick, F. The RB and p53 pathways in cancer. Cancer Cell 2, 103–112 (2002)
Chau, B. N. & Wang, J. Y. Coordinated regulation of life and death by RB. Nature Rev. Cancer 3, 130–138 (2003)
Vogelstein, B., Lane, D. & Levine, A. J. Surfing the p53 network. Nature 408, 307–310 (2000)
Oren, M. Decision making by p53: life, death and cancer. Cell Death Differ. 10, 431–442 (2003)
Prives, C. & Hall, P. A. The p53 pathway. J. Pathol. 187, 112–126 (1999)
Honda, R., Tanaka, H. & Yasuda, H. Oncoprotein MDM2 is a ubiquitin ligase E3 for tumor suppressor p53. FEBS Lett. 420, 25–27 (1997)
Kubbutat, M. H., Jones, S. N. & Vousden, K. H. Regulation of p53 stability by Mdm2. Nature 387, 299–303 (1997)
Momand, J., Jung, D., Wilczynski, S. & Niland, J. The MDM2 gene amplification database. Nucleic Acids Res. 26, 3453–3459 (1998)
Yang, Y. et al. Small molecule inhibitors of HDM2 ubiquitin ligase activity stabilize and activate p53 in cells. Cancer Cell 7, 547–559 (2005)
Vassilev, L. T. et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 303, 844–848 (2004)
Kato, M. V. et al. Loss of heterozygosity on chromosome 17 and mutation of the p53 gene in retinoblastoma. Cancer Lett. 106, 75–82 (1996)
Nork, T. M., Poulsen, G. L., Millecchia, L. L., Jantz, R. G. & Nickells, R. W. p53 regulates apoptosis in human retinoblastoma. Arch. Ophthalmol. 115, 213–219 (1997)
Chen, D. et al. Cell-specific effects of RB or RB/p107 loss on retinal development implicate an intrinsically death-resistant cell-of-origin in retinoblastoma. Cancer Cell 5, 539–551 (2004)
Dyer, M. A. & Bremner, R. The search for the retinoblastoma cell of origin. Nature Rev. Cancer 5, 91–101 (2005)
Aslanian, A., Iaquinta, P. J., Verona, R. & Lees, J. A. Repression of the Arf tumor suppressor by E2F3 is required for normal cell cycle kinetics. Genes Dev. 18, 1413–1422 (2004)
Lowe, S. W. & Sherr, C. J. Tumor suppression by Ink4a-Arf: progress and puzzles. Curr. Opin. Genet. Dev. 13, 77–83 (2003)
Zhang, J., Schweers, B. & Dyer, M. A. The first knockout mouse model of retinoblastoma. Cell Cycle 3, 952–959 (2004)
Donovan, S., Schweers, B., Martins, R., Johnson, D. & Dyer, M. A. Compensation by tumor suppressor genes during retinal development in mice and humans. BMC Biol. 4, 14 (2006)
Shvarts, A. et al. MDMX: a novel p53-binding protein with some functional properties of MDM2. EMBO J. 15, 5349–5357 (1996)
Danovi, D. et al. Amplification of Mdmx (or Mdm4) directly contributes to tumor formation by inhibiting p53 tumor suppressor activity. Mol. Cell. Biol. 24, 5835–5843 (2004)
McKenzie, P. P., McPake, C. R., Ashford, A. A., Vanin, E. F. & Harris, L. C. MDM2 does not influence p53-mediated sensitivity to DNA-damaging drugs. Mol. Cancer Ther. 1, 1097–1104 (2002)
Canman, C. E. et al. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science 281, 1677–1679 (1998)
Kastan, M. B., Lim, D. S., Kim, S. T. & Yang, D. ATM—a key determinant of multiple cellular responses to irradiation. Acta Oncol. 40, 686–688 (2001)
Kastan, M. B., Onyekwere, O., Sidransky, D., Vogelstein, B. & Craig, R. W. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 51, 6304–6311 (1991)
Brummelkamp, T. R., Bernards, R. & Agami, R. A system for stable expression of short interfering RNAs in mammalian cells. Science 296, 550–553 (2002)
Baker, S. J., Markowitz, S., Fearon, E. R., Willson, J. K. & Vogelstein, B. Suppression of human colorectal carcinoma cell growth by wild-type p53. Science 249, 912–915 (1990)
Robanus-Maandag, E. et al. p107 is a suppressor of retinoblastoma development in pRb-deficient mice. Genes Dev. 12, 1599–1609 (1998)
MacPherson, D. et al. Cell type-specific effects of Rb deletion in the murine retina. Genes Dev. 18, 1681–1694 (2004)
Dyer, M. A. & Harbour, J. W. in Clinical Ocular Oncology Ch. 66 (eds Singh, A. D., Damato, B., Murphree, A. L. & Perry, J. D.) (Elsevier, London, in the press).
Dyer, M. A., Rodriguez-Galindo, C. & Wilson, M. W. Use of preclinical models to improve treatment of retinoblastoma. PLoS Med. 2, e332 (2005)
Marquardt, T. et al. Pax6 is required for the multipotent state of retinal progenitor cells. Cell 105, 43–55 (2001)
Lu, F. et al. Proteomimetic libraries: design, synthesis, and evaluation of p53–MDM2 interaction inhibitors. J. Comb. Chem. 8, 315–325 (2006)
Dang, J. et al. The RING domain of Mdm2 can inhibit cell proliferation. Cancer Res. 62, 1222–1230 (2002)
Laurie, N. A. et al. Topotecan combination chemotherapy in two new rodent models of retinoblastoma. Clin. Cancer Res. 11, 7569–7578 (2005)
Acknowledgements
We thank L. Harris, G. Zambetti and M. Baron for discussions; S. Pounds for statistical analysis; M. Roussel for MDMX and MDM2 retroviruses; B. Schulman and D. Bashford for assistance with MDMX–nutlin-3 modelling; A. McArthur for editorial assistance; J. Gray for assistance with real-time RT–PCR and genomic DNA preparations; and F. Carlotti and M. Rabeling for advice on lentiviral experiments and production of lentivirus stocks. This work was supported by grants (to M.A.D.) from the National Eye Institute, Cancer Center Support from the National Cancer Institute, the American Cancer Society, Research to Prevent Blindness, the Pearle Vision Foundation, the International Retinal Research Foundation and the American Lebanese Syrian Associated Charities (ALSAC). M.A.D. is a Pew Scholar. This work was also supported by funding from the Association for International Cancer Research (A.G.J.) and EC FP6 (A.G.J. and J.-C.M.), the Dutch Cancer Society (Y.R.), the Belgian Foundation against Cancer (J.-C.M.) and Télévie (S.F.). This publication was supported in part by a grant from the National Cancer Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests.
Supplementary information
Supplementary Notes
This file contains Supplementary Tables 1–8, Supplementary Figures 1–10 and detailed Supplementary Methods. The Supplementary Tables present data from dissociated cell scoring, FISH analysis and clone size analysis of MDM2Lox/Lox MEFs. The Supplementary Figures provide additional data on modulation of the p53 pathway by MDM2 and MDMX in normal and transformed cells. The figures also provide additional data on the mechanism of topotecan and nutlin-3 mediated killing of retinoblastoma cells and cell-based assays demonstrating that nutlin-3 can kill Mdm2-deficient MEFs by binding to MdmX. The Supplementary Methods provide details about antibodies, cDNAs, siRNAs, microscopy, retinoblastoma tissue analysis, statistical analysis, protein purification and nutlin-3 binding studies and MEF experiments. (PDF 5104 kb)
Rights and permissions
About this article
Cite this article
Laurie, N., Donovan, S., Shih, CS. et al. Inactivation of the p53 pathway in retinoblastoma. Nature 444, 61–66 (2006). https://doi.org/10.1038/nature05194
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature05194
This article is cited by
-
Retinoblastoma: present scenario and future challenges
Cell Communication and Signaling (2023)
-
1H, 15N and 13C backbone resonance assignments of the acidic domain of the human MDMX protein
Biomolecular NMR Assignments (2022)
-
MDM4: What do we know about the association between its polymorphisms and cancer?
Medical Oncology (2022)
-
Resistance mechanisms to inhibitors of p53-MDM2 interactions in cancer therapy: can we overcome them?
Cellular & Molecular Biology Letters (2021)
-
Retinoblastoma tumor cell proliferation is negatively associated with an immune gene expression signature and increased immune cells
Laboratory Investigation (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.