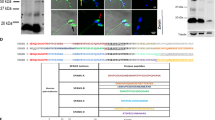
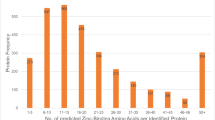
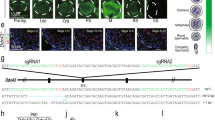
Abstract
Male infertility is a long-standing enigma of significant medical concern. The integrity of sperm chromatin is a clinical indicator of male fertility and in vitro fertilization potential1: chromosome aneuploidy and DNA decondensation or damage are correlated with reproductive failure. Identifying conserved proteins important for sperm chromatin structure and packaging can reveal universal causes of infertility. Here we combine proteomics, cytology and functional analysis in Caenorhabditis elegans to identify spermatogenic chromatin-associated proteins that are important for fertility. Our strategy employed multiple steps: purification of chromatin from comparable meiotic cell types, namely those undergoing spermatogenesis or oogenesis; proteomic analysis by multidimensional protein identification technology (MudPIT) of factors that co-purify with chromatin; prioritization of sperm proteins based on abundance; and subtraction of common proteins to eliminate general chromatin and meiotic factors. Our approach reduced 1,099 proteins co-purified with spermatogenic chromatin, currently the most extensive catalogue, to 132 proteins for functional analysis. Reduction of gene function through RNA interference coupled with protein localization studies revealed conserved spermatogenesis-specific proteins vital for DNA compaction, chromosome segregation, and fertility. Unexpected roles in spermatogenesis were also detected for factors involved in other processes. Our strategy to find fertility factors conserved from C. elegans to mammals achieved its goal: of mouse gene knockouts corresponding to nematode proteins, 37% (7/19) cause male sterility. Our list therefore provides significant opportunity to identify causes of male infertility and targets for male contraceptives.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Agarwal, A. & Said, T. M. Role of sperm chromatin abnormalities and DNA damage in male infertility. Hum. Reprod. Update 9, 331–345 (2003)
Kimmins, S. & Sassone-Corsi, P. Chromatin remodelling and epigenetic features of germ cells. Nature 434, 583–589 (2005)
Washburn, M. P., Wolters, D. & Yates, J. R. Large-scale analysis of the yeast proteome by multidimensional protein identification technology. Nature Biotechnol. 19, 242–247 (2001)
Eng, J. K., McCormack, A. & Yates, J. R. An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J. Am. Soc. Mass Spectrom. 5, 976–989 (1994)
Tabb, D. L., McDonald, W. H. & Yates, J. R. DTASelect and Contrast: tools for assembling and comparing protein identifications from shotgun proteomics. J. Proteome Res. 1, 21–26 (2002)
Schirmer, E. C., Florens, L., Guan, T., Yates, J. R. & Gerace, L. Nuclear membrane proteins with potential disease links found by subtractive proteomics. Science 301, 1380–1382 (2003)
Skop, A. R., Liu, H., Yates, J., Meyer, B. J. & Heald, R. Dissection of the mammalian midbody proteome reveals conserved cytokinesis mechanisms. Science 305, 61–66 (2004)
Browning, H. & Strome, S. A sperm-supplied factor required for embryogenesis in C. elegans. Development 122, 391–404 (1996)
Lee, M. H., Park, H., Shim, G., Lee, J. & Koo, H. S. Regulation of gene expression, cellular localization, and in vivo function of Caenorhabditis elegans DNA topoisomerase I. Genes Cells 6, 303–312 (2001)
Gruidl, M. E. et al. Multiple potential germ-line helicases are components of the germ-line-specific P granules of Caenorhabditis elegans. Proc. Natl Acad. Sci. USA 93, 13837–13842 (1996)
Moore, L. L. & Roth, M. B. HCP-4, a CENP-C like protein in Caenorhabditis elegans, is required for resolution of sister centromeres. J. Cell Biol. 153, 1199–1208 (2001)
Reinke, V., Gil, I., Ward, S. & Kazmer, K. Genome-wide germline-enriched and sex-biased expression profiles in Caenorhabditis elegans. Development 131, 311–323 (2004)
Kamath, R. S. et al. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 421, 231–237 (2003)
Sonnichsen, B. et al. Full genome RNAi profiling of early embryogenesis in Caenorhabditis elegans. Nature 434, 462–469 (2005)
Varmuza, S. et al. Spermiogenesis is impaired in mice bearing a targeted mutation in the protein phosphatase 1c gamma gene. Dev. Biol. 205, 98–110 (1999)
Boag, P. R., Ren, P., Newton, S. E. & Gasser, R. B. Molecular characterisation of a male specific serine/threonine phosphatase from Oesophagostomum dentatum (Nematoda: Strongylida), and functional analysis of homologues in Caenorhabditis elegans. Int. J. Parasitol. 33, 313–325 (2003)
Hanazawa, M., Mochii, M., Ueno, N., Kohara, Y. & Iino, Y. Use of cDNA subtraction and RNA interference screens in combination. Proc. Natl Acad. Sci. USA 98, 8686–8691 (2001)
Rogers, E., Bishop, J. D., Waddle, J. A., Schumacher, J. M. & Lin, R. The aurora kinase AIR-2 functions in the release of chromosome cohesion in Caenorhabditis elegans meiosis. J. Cell Biol. 157, 219–229 (2002)
Celeste, A. et al. Genomic instability in mice lacking histone H2AX. Science 296, 922–927 (2002)
Lewis, J. D., McParland, R. & Ausio, J. PL I of Spisula solidissima, a highly elongated sperm specific histone H1. Biochemistry 43, 7766–7775 (2004)
Lewis, J. D., Song, Y., de Jong, M. E., Bagha, S. M. & Ausio, J. A walk though vertebrate and invertebrate protamines. Chromosoma 111, 473–482 (2003)
Martianov, I. et al. Polar nuclear localization of H1T2, a histone H1 variant, required for spermatid elongation and DNA condensation during spermiogenesis. Proc. Natl Acad. Sci. USA 102, 2808–2813 (2005)
Swanson, W. J. & Vacquier, V. D. The rapid evolution of reproductive proteins. Nature Rev. Genet. 3, 137–144 (2002)
Wang, J. C. Cellular roles of DNA topoisomerases: a molecular perspective. Nature Rev. Mol. Cell Biol. 3, 430–440 (2002)
Rossi, F. et al. Specific phosphorylation of SR proteins by mammalian DNA topoisomerase I. Nature 381, 80–82 (1996)
Kawano, T., Fujita, M. & Sakamoto, H. Unique and redundant functions of SR proteins, a conserved family of splicing factors, in Caenorhabditis elegans development. Mech. Dev. 95, 67–76 (2000)
Tanaka, S. S. et al. The mouse homolog of Drosophila Vasa is required for the development of male germ cells. Genes Dev. 14, 841–853 (2000)
Vogt, P. H. Azoospermia factor (AZF) in Yq11: towards a molecular understanding of its function for human male fertility and spermatogenesis. Reprod. Biomed. Online 10, 81–93 (2005)
Ward, S. & Klass, M. The location of the major protein in Caenorhabditis elegans sperm and spermatocytes. Dev. Biol. 92, 203–208 (1982)
Liu, H., Sadygov, R. G. & Yates, J. R. A model for random sampling and estimation of relative protein abundance. Anal. Chem. 76, 4193–4201 (2004)
Acknowledgements
We thank S. Strome, K. Bennett, H.-S. Koo, L. Moore, E. Shulze and J. Aris for providing antibodies; A. Villenueve, G. Stanfield, S. Mitani and the C. elegans Genetic Center (CGC) for providing strains; V. Reinke, L. Moore and R. Navarro for sharing unpublished data; D. King for peptide synthesis; A. Chan for help with microscopy; S. Chu for statistical analysis; A. Severson, T. Cline, A. Skop, E. Xu and J. Gladden for comments on the manuscript; and members of the Meyer laboratory for input on this project. This work was funded by the National Institutes of Health grants to D.S.C., J.R.Y. and B.J.M. B.J.M. is an investigator of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests.
Supplementary information
Supplementary Notes
This file contains Supplementary Methods that contain additional detailed methods used in the paper, Supplementary references, legends for Supplementary Figs 1–5 and a legend for Supplementary Table 6. (DOC 112 kb)
Supplementary Figures
This file contains Supplementary figures 1–5. (PDF 1188 kb)
Supplementary Table 1
Abundant spermatogenesis-enriched proteins copurified with chromatin identified by comparative proteomic analysis. (PDF 68 kb)
Supplementary Table 2
Low abundance spermatogenic proteins copurified with chromatin identified by comparative proteomic analysis (PDF 102 kb)
Supplementary Table 3
All shared proteins copurified with chromatin identified by comparative proteomic analysis. (PDF 139 kb)
Supplementary Table 4
Oogenic proteins copurified with chromatin identified by comparative proteomic analysis. (PDF 87 kb)
Supplementary Table 5
This table lists the composition of identified proteins from spermatogenic chromatin samples in functional categories. (PDF 34 kb)
Supplementary Table 6
This table summarizes defects for genes found to be important for fertility by RNAi analysis. (PDF 61 kb)
Supplementary Table 7
This table lists abundant spermatogenesis-enriched chromatin proteins with function in C. elegans fertility. The protein localization has not yet been determined in C. elegans (Category III proteins). Mouse and human homologs with a minimum E value of 1e-9 are listed. (PDF 62 kb)
Supplementary Table 8
The table lists abundant C. elegans spermatogenesis-enriched chromatin proteins homologous to mammalian fertility factors. Mouse and human homologs with a minimum E value of 1e-9 are listed. (PDF 62 kb)
Supplementary Table 9
This table lists abundant C. elegans spermatogenesis-enriched chromatin proteins with mammalian homologs not yet linked to fertility. Mouse and human homologs with a minimum E value of 1e-9 are listed. (PDF 80 kb)
Rights and permissions
About this article
Cite this article
Chu, D., Liu, H., Nix, P. et al. Sperm chromatin proteomics identifies evolutionarily conserved fertility factors. Nature 443, 101–105 (2006). https://doi.org/10.1038/nature05050
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature05050
This article is cited by
-
Expression of Heterorhabditis bacteriophora C-type lectins, Hb-clec-1 and Hb-clec-78, in context of symbiosis with Photorhabdus bacteria
Symbiosis (2019)
-
Leagues of their own: sexually dimorphic features of meiotic prophase I
Chromosoma (2019)
-
In-depth proteomic analysis of boar spermatozoa through shotgun and gel-based methods
BMC Genomics (2018)
-
The sperm factor: paternal impact beyond genes
Heredity (2018)
-
Caenorhabditis elegans sperm carry a histone-based epigenetic memory of both spermatogenesis and oogenesis
Nature Communications (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.