Abstract
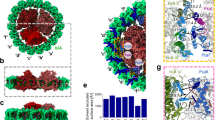
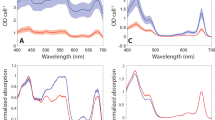
Adjustment of catalytic activity in response to diverse ambient temperatures is fundamental to life on Earth. A crucial example of this is photosynthesis, where solar energy is converted into electrochemical potential that drives oxygen and biomass generation at temperatures ranging from those of frigid Antarctica to those of scalding hot springs. The energy conversion proceeds by concerted mobilization of electrons and protons on photoexcitation of reaction centre protein complexes1,2,3. Following physicochemical paradigms, the rates of imperative steps in this process were predicted to increase exponentially with rising temperatures, resulting in different yields of solar energy conversion at the distinct growth temperatures of photosynthetic mesophiles and extremophiles. In contrast, here we show a meticulous adjustment of energy conversion rate, resulting in similar yields from mesophiles and thermophiles. The key molecular players in the temperature adjustment process consist of a cluster of hitherto unrecognized protein cavities and an adjacent packing motif that jointly impart local flexibility crucial to the reaction centre proteins. Mutations within the packing motif of mesophiles that increase the bulkiness of the amino-acid side chains, and thus reduce the size of the cavities, promote thermophilic behaviour. This novel biomechanical mechanism accounts for the slowing of the catalytic reaction above physiological temperatures in contradiction to the classical Arrhenius paradigm. The mechanism provides new guidelines for manipulating the acclimatization of enzymes to the ambient temperatures of diverse habitats. More generally, it reveals novel protein elements that are of potential significance for modulating structure–activity relationships in membrane and globular proteins alike.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Okamura, M. Y., Paddock, M. L., Graige, M. S. & Feher, G. Proton and electron transfer in bacterial reaction centers. Biochim. Biophys. Acta 1458, 148–163 (2000)
Iwata, S. & Barber, J. Structure of photosystem II and molecular architecture of the oxygen-evolving centre. Curr. Opin. Struct. Biol. 14, 447–453 (2004)
Wraight, C. A. Proton and electron transfer in the acceptor quinone complex of photosynthetic reaction centers from Rhodobacter sphaeroides. Front. Biosci. 9, 309–337 (2004)
Ferreira, K. N., Iverson, T. M., Maghlaoui, K., Barber, J. & Iwata, S. Architecture of the photosynthetic oxygen-evolving center. Science 303, 1831–1838 (2004)
Loll, B., Kern, J., Saenger, W., Zouni, A. & Biesiadka, J. Towards complete cofactor arrangement in the 3.0 Å resolution structure of photosystem II. Nature 438, 1040–1044 (2005)
Moser, C. C. et al. in Advances in Protein Chemistry 63: Membrane Proteins (ed. Rees, D. C.) 71–109 (Academic, San Diego, 2003)
Li, J., Gilroy, D., Tiede, D. M. & Gunner, M. R. Kinetic phases in the electron transfer from P+QA-QB to P+QAQB- and the associated processes in Rhodobacter sphaeroides R-26 reaction centers. Biochemistry 37, 2818–2829 (1998)
Garbers, A., Reifarth, F., Kurreck, J., Renger, G. & Parak, F. Correlation between protein flexibility and electron transfer from QA- • to QB in PSII membrane fragments from spinach. Biochemistry 37, 11399–11404 (1998)
Xu, Q. & Gunner, M. R. Exploring the energy profile of the QA- to QB electron transfer reaction in bacterial photosynthetic reaction centers: pH dependence of the conformational gating step. Biochemistry 41, 2694–2701 (2002)
Joshi, M. & Fragata, M. Heat-induced changes in the photochemical centres and the protein secondary structures of photosystem II studied by variable fluorescence and difference FT-IR spectroscopy. Z. Naturforsch. 54, 35–43 (1999)
Deisenhofer, J., Epp, O., Sinning, I. & Michel, H. Crystallographic refinement at 2.3 Å resolution and refined model of the photosynthetic reaction centre from Rhodopseudomonas viridis. J. Mol. Biol. 246, 429–457 (1995)
Senes, A., Engel, D. E. & DeGrado, W. F. Folding of helical membrane proteins: the role of polar, GxxxG-like and proline motifs. Curr. Opin. Struct. Biol. 14, 465–479 (2004)
Curran, A. R. & Engelman, D. M. Sequence motifs, polar interactions and conformational changes in helical membrane proteins. Curr. Opin. Struct. Biol. 13, 412–417 (2003)
Friedman, R., Nachliel, E. & Gutman, M. The role of small intraprotein cavities in the catalytic cycle of bacteriorhodopsin. Biophys. J. 85, 886–896 (2003)
Eilers, M., Shekar, S., Shieh, T., Smith, S. & Fleming, P. Internal packing of helical membrane proteins. Proc. Natl Acad. Sci. USA 97, 5796–5801 (2000)
Knapp, M. J., Rickert, K. & Klinman, J. P. Temperature-dependent isotope effects in soybean lipoxygenase-1: Correlating hydrogen tunneling with protein dynamics. J. Am. Chem. Soc. 124, 3865–3874 (2002)
Kohen, A., Cannio, R., Bartolucci, S. & Klinman, J. P. Enzyme dynamics and hydrogen tunnelling in a thermophilic alcohol dehydrogenase. Nature 399, 496–499 (1999)
Liang, Z. X., Lee, T., Resing, K. A., Ahn, N. G. & Klinman, J. P. Thermal-activated protein mobility and its correlation with catalysis in thermophilic alcohol dehydrogenase. Proc. Natl Acad. Sci. USA 101, 9556–9561 (2004)
Wong, K. F., Selzer, T., Benkovic, S. J. & Hammes-Schiffer, S. Impact of distal mutations on the network of coupled motions correlated to hydride transfer in dihydrofolate reductase. Proc. Natl Acad. Sci. USA 102, 6807–6812 (2005)
Kohen, A. & Klinman, J. P. Enzyme catalysis: Beyond classical paradigms. Acc. Chem. Res. 31, 397–404 (1998)
Hammes-Schiffer, S. & Iordanova, N. Theoretical studies of proton-coupled electron transfer reactions. Biochim. Biophys. Acta 1655, 29–36 (2004)
Remy, A. & Gerwert, K. Coupling of light-induced electron transfer to proton uptake in photosynthesis. Nature Struct. Biol. 10, 637–644 (2003)
Somero, G. N. Protein adaptations to temperature and pressure: complementary roles of adaptive changes in amino-acid sequence and internal milieu. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 136, 577–591 (2003)
Závodszky, P., Kardos, J., Svingor, Á. & Petsko, G. A. Adjustment of conformational flexibility is a key event in the thermal adaptation of proteins. Proc. Natl Acad. Sci. USA 95, 7406–7411 (1998)
Bower, M. J., Cohen, F. E. & Dunbrack, R. L. Jr . Prediction of protein side-chain rotamers from a backbone-dependent rotamer library: a new homology modeling tool. J. Mol. Biol. 267, 1268–1282 (1997)
Word, J. M. et al. Visualizing and quantifying molecular goodness-of-fit: small-probe contact dots with explicit hydrogen atoms. J. Mol. Biol. 285, 1711–1733 (1999)
Senes, A., Ubarretxena-Belandia, I. & Engelman, D. M. The Cα—H···O hydrogen bond: A determinant of stability and specificity in transmembrane helix interactions. Proc. Natl. Acad. Sci. USA 98, 9056–9061 (2001)
Goldberg, E. Membrane Protein Assembly: The Role of Backbone Mediated Interhelical Hydrogen Bonds. Master's thesis, Weizmann Institute of Science (2004)
Liang, J., Edelsbrunner, H., Fu, P., Sudhakar, P. V. & Subramaniam, S. Analytical shape computation of macromolecules: II. Inaccessible cavities in proteins. Proteins 33, 18–29 (1998)
Nedbal, L., Trtílek, M. & Kaftan, D. Flash fluorescence induction: a novel method to study regulation of photosystem II. J. Photochem. Photobiol. B 48, 154–157 (1999)
Acknowledgements
We thank B. Loll, J. Biesiadka, and W. Saenger for sending us their PS II reaction centre coordinates before publication; M. Edelman for critical reading of the manuscript; S. Pietrokovski for assistance in data mining sequences; and W. L. DeLano for assistance in applying new PyMol methods. This research was supported by a Sonderforschungsbereich grant and the Willstatter-Avron-Minerva Foundation for Photosynthesis. D.K. was also supported by the Ministry of Education, Youth, and Sports of the Czech Republic; the Academy of Sciences of the Czech Republic; and by the NIH NBCR. A.S. is the incumbent of the Yadelle and Robert Sklare Professorial Chair for Biochemistry. Author Contributions A.S. carried out project planning and supervision. O.S. designed and performed mutagenesis. O.S. and D.K. performed experimental work, mainly at the WIS and partly at USB and ACSR. O.S., D.K. and I.S. performed comparative sequence analysis. I.S. performed the in silico study. O.S., D.K., P.S.M.S., I.S. and A.S. carried out experimental data analysis. H.K. and N.H. helped to establish the mutagenesis program. The study was done in partial fulfilment of the PhD theses of O.S. and I.S.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
Supplementary information
Supplementary Notes
This file contains Supplementary Materials, Supplementary Figure and Supplementary Tables. (DOC 1630 kb)
Rights and permissions
About this article
Cite this article
Shlyk-Kerner, O., Samish, I., Kaftan, D. et al. Protein flexibility acclimatizes photosynthetic energy conversion to the ambient temperature. Nature 442, 827–830 (2006). https://doi.org/10.1038/nature04947
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature04947
This article is cited by
-
Targeting mutations to the plastidial psbA gene of Chlamydomonas reinhardtii without direct positive selection
Scientific Reports (2019)
-
Temperature dependence of photosynthetic reaction centre activity in Rhodospirillum rubrum
Photosynthesis Research (2019)
-
Photosystem-II D1 protein mutants of Chlamydomonas reinhardtii in relation to metabolic rewiring and remodelling of H-bond network at QB site
Scientific Reports (2018)
-
Rate-limiting steps in the dark-to-light transition of Photosystem II - revealed by chlorophyll-a fluorescence induction
Scientific Reports (2018)
-
A single residue controls electron transfer gating in photosynthetic reaction centers
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.