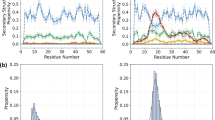
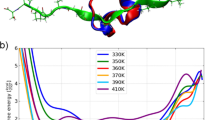
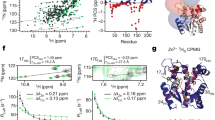
Abstract
Protein folding is an inherently complex process involving coordination of the intricate networks of weak interactions that stabilize native three-dimensional structures. In the conventional paradigm, simple protein structures are assumed to fold in an all-or-none process1 that is inaccessible to experiment. Existing experimental methods therefore probe folding mechanisms indirectly. A widely used approach interprets changes in protein stability2 and/or folding kinetics3,4, induced by engineered mutations, in terms of the structure of the native protein. In addition to limitations in connecting energetics with structure5, mutational methods have significant experimental uncertainties6 and are unable to map complex networks of interactions. In contrast, analytical theory predicts small barriers to folding and the possibility of downhill folding7,8. These theoretical predictions have been confirmed experimentally in recent years9,10,11, including the observation of global downhill folding12. However, a key remaining question is whether downhill folding can indeed lead to the high-resolution analysis of protein folding processes13. Here we show, with the use of nuclear magnetic resonance (NMR), that the downhill protein BBL from Escherichia coli unfolds atom by atom starting from a defined three-dimensional structure. Thermal unfolding data on 158 backbone and side-chain protons out of a total of 204 provide a detailed view of the structural events during folding. This view confirms the statistical nature of folding, and exposes the interplay between hydrogen bonding, hydrophobic forces, backbone conformation and side-chain entropy. From the data we also obtain a map of the interaction network in this protein, which reveals the source of folding cooperativity. Our approach can be extended to other proteins with marginal barriers (less than 3RT), providing a new tool for the study of protein folding.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Tanford, C. Protein denaturation. Adv. Prot. Chem. 23, 121–282 (1968)
Matthews, B. W. Structural and genetic-analysis of protein-folding and stability. Curr. Opin. Struct. Biol. 3, 589–593 (1993)
Goldenberg, D. P. in Protein Folding (ed. Cheighton, T. E.) 353–406 (Freeman, New York, 1992)
Fersht, A. R., Matouschek, A. & Serrano, L. The folding of an enzyme. 1. Theory of protein engineering analysis of stability and pathway of protein folding. J. Mol. Biol. 224, 771–782 (1992)
Honig, B. & Yang, A. S. Free-energy balance in protein-folding. Adv. Protein Chem. 46, 27–58 (1995)
Sanchez, I. E. & Kiefhaber, T. Origin of unusual φ-values in protein folding: Evidence against specific nucleation sites. J. Mol. Biol. 334, 1077–1085 (2003)
Bryngelson, J. D., Onuchic, J. N., Socci, N. D. & Wolynes, P. G. Funnels, pathways, and the energy landscape of protein-folding—a synthesis. Prot. Struct. Funct. Genet. 21, 167–195 (1995)
Onuchic, J. N., LutheySchulten, Z. & Wolynes, P. G. Theory of protein folding: The energy landscape perspective. Annu. Rev. Phys. Chem. 48, 545–600 (1997)
Yang, W. Y. & Gruebele, M. Folding at the speed limit. Nature 423, 193–197 (2003)
Kubelka, J., Hofrichter, J. & Eaton, W. A. The protein folding ‘speed limit’. Curr. Opin. Struct. Biol. 14, 76–88 (2004)
Akmal, A. & Muñoz, V. The nature of the free energy barriers to two-state folding. Prot. Struct. Funct. Bioinformatics 57, 142–152 (2004)
Garcia-Mira, M. M., Sadqi, M., Fischer, N., Sanchez-Ruiz, J. M. & Muñoz, V. Experimental identification of downhill protein folding. Science 298, 2191–2195 (2002)
Muñoz, V. Thermodynamics and kinetics of downhill protein folding investigated with a simple statistical mechanical model. Int. J. Quantum Chem. 90, 1522–1528 (2002)
Oliva, F. Y. & Muñoz, V. A simple thermodynamic test to discriminate between two-state and downhill folding. J. Am. Chem. Soc. 126, 8596–8597 (2004)
Muñoz, V. & Sanchez-Ruiz, J. M. Exploring protein folding ensembles: A variable barrier model for the analysis of equilibrium unfolding experiments. Proc. Natl Acad. Sci. USA 101, 17646–17651 (2004)
Robien, M. A. et al. 3-Dimensional solution structure of the E3-binding domain of the dihydrolipoamide succinyl transferase core from the 2-oxoglutarate dehydrogenase multienzyme complex of Escherichia coli.. Biochemistry 31, 3463–3471 (1992)
Naganathan, A. N., Perez-Jimenez, R., Sanchez-Ruiz, J. M. & Muñoz, V. Robustness of downhill folding: Guidelines for the analysis of equilibrium folding experiments on small proteins. Biochemistry 44, 7435–7449 (2005)
Hanggi, P., Talkner, P. & Borkovec, M. Reaction-rate theory: Fifty years after Kramers. Rev. Mod. Phys. 62, 251–341 (1990)
Maity, H., Maity, M., Krishna, M. M. G., Mayne, L. & Englander, S. W. Protein folding: The stepwise assembly of foldon units. Proc. Natl Acad. Sci. USA 102, 4741–4746 (2005)
Wagner, G., Pardi, A. & Wüthrich, K. Hydrogen-bond length and H-1-NMR chemical-shifts in proteins. J. Am. Chem. Soc. 105, 5948–5949 (1983)
Wishart, D. S. & Sykes, B. D. Chemical-shifts as a tool for structure determination. Nucl. Magn. Res. C 239, 363–392 (1994)
Arai, M. & Kuwajima, K. Rapid formation of a molten globule intermediate in refolding of α-lactalbumin. Fold. Des. 1, 275–287 (1996)
Plotkin, S. S., Wang, J. & Wolynes, P. G. Statistical mechanics of a correlated energy landscape model for protein folding funnels. J. Chem. Phys. 106, 2932–2948 (1997)
Shimizu, S. & Chan, H. S. Anti-cooperativity and cooperativity in hydrophobic interactions: Three-body free energy landscapes and comparison with implicit-solvent potential functions for proteins. Prot. Struct. Funct. Genet. 48, 15–30 (2002)
Naganathan, A. N. & Muñoz, V. Scaling of folding times with protein size. J. Am. Chem. Soc. 127, 480–481 (2005)
Holtzer, M. E., Lovett, E. G., d'Avignon, D. A. & Holtzer, A. Thermal unfolding in a GCN4-like leucine zipper: C-13α NMR chemical shifts and local unfolding curves. Biophys. J. 73, 1031–1041 (1997)
Klimov, D. K. & Thirumalai, D. Is there a unique melting temperature for two-state proteins? J. Comp. Chem. 23, 161–165 (2002)
Palmer, A. G., Kroenke, C. D. & Loria, J. P. Nuclear magnetic resonance methods for quantifying microsecond-to-millisecond motions in biological macromolecules. Methods Enzymol. 339, 204–238 (2001)
Tollinger, M., Skrynnikov, N. R., Mulder, F. A., Forman-Kay, J. D. & Kay, L. E. Slow dynamics in folded and unfolded states on an SH3 domain. J. Am. Chem. Soc. 123, 11341–11352 (2001)
Acknowledgements
We thank E. de Alba for help and guidance in the calculation of NMR structures with Xplor-NIH and for generating the table of structure statistics; P. Vasos for assistance with the NMR relaxation experiments; and Y. Hathout for mass-spectrometric analysis. The research described in this article was supported by the NIH and the NSF. Author Contributions D.F. performed and analysed the NMR relaxation experiments and contributed to manuscript preparation. M.S. performed all other NMR experiments and circular dichroism experiments, assigned all the NMR spectra, and contributed to manuscript preparation. V.M. conceived the project, supervised the work, developed the methods for analysis, analysed the experimental data, and contributed to manuscript preparation. The structural analysis was performed in conjunction by M.S. and V.M.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The atomic coordinates of Naf-BBL have been deposited in the Protein Data Bank with the accession number 2QYU. Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
Supplementary information
Supplementary Notes
This file contains six sections. The first section of the contains a description of the determination of the NMR structure at 278 K, including a Supplementary Table with the structure statistics. The second section contains a more detailed description of the NMR relaxation experiments. The third section includes additional technical information about the analysis of chemical shift versus temperature curves in terms of atomic folding-unfolding equilibria. The fourth section contains the equations and formalism for calculation of the mean thermodynamic coupling index (MTCI) and matrix of residue-residue mean thermodynamic couplings from chemical shift versus temperature unfolding curves. The fifth section contains a technical description of the methods employed in this work.The sixth section is a table of the chemical shifts versus temperature for the 158 protons of the protein BBL that have been employed in this work. (DOC 5586 kb)
Rights and permissions
About this article
Cite this article
Sadqi, M., Fushman, D. & Muñoz, V. Atom-by-atom analysis of global downhill protein folding. Nature 442, 317–321 (2006). https://doi.org/10.1038/nature04859
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature04859
This article is cited by
-
Monitoring protein unfolding transitions by NMR-spectroscopy
Journal of Biomolecular NMR (2022)
-
The change of conditions does not affect Ros87 downhill folding mechanism
Scientific Reports (2020)
-
Reversible two-state folding of the ultrafast protein gpW under mechanical force
Communications Chemistry (2018)
-
A simple two-state protein unfolds mechanically via multiple heterogeneous pathways at single-molecule resolution
Nature Communications (2016)
-
Long range Trp-Trp interaction initiates the folding pathway of a pro-angiogenic β-hairpin peptide
Scientific Reports (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.