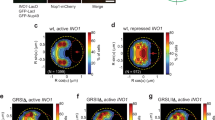
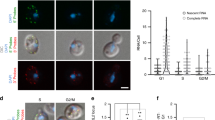
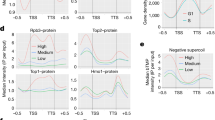
Abstract
The organization of the nucleus into subcompartments creates microenvironments that are thought to facilitate distinct nuclear functions1. In budding yeast, regions of silent chromatin, such as those at telomeres and mating-type loci, cluster at the nuclear envelope creating zones that favour gene repression1,2. Other reports indicate that gene transcription occurs at the nuclear periphery, apparently owing to association of the gene with nuclear pore complexes3,4,5. Here we report that transcriptional activation of a subtelomeric gene, HXK1 (hexokinase isoenzyme 1), by growth on a non-glucose carbon source led to its relocalization to nuclear pores. This relocation required the 3′ untranslated region (UTR), which is essential for efficient messenger RNA processing and export, consistent with an accompanying report6. However, activation of HXK1 by an alternative pathway based on the transactivator VP16 moved the locus away from the nuclear periphery and abrogated the normal induction of HXK1 by galactose. Notably, when we interfered with HXK1 localization by either antagonizing or promoting association with the pore, transcript levels were reduced or enhanced, respectively. From this we conclude that nuclear position has an active role in determining optimal gene expression levels.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Taddei, A., Hediger, F., Neumann, F. R. & Gasser, S. M. The function of nuclear architecture: a genetic approach. Annu. Rev. Genet. 38, 305–345 (2004)
Andrulis, E. D., Neiman, A. M., Zappulla, D. C. & Sternglanz, R. Perinuclear localization of chromatin facilitates transcriptional silencing. Nature 395, 592–595 (1998)
Casolari, J. M. et al. Genome-wide localization of the nuclear transport machinery couples transcriptional status and nuclear organization. Cell 117, 427–439 (2004)
Brickner, J. H. & Walter, P. Gene recruitment of the activated INO1 locus to the nuclear membrane. PLoS Biol. 2, e342 (2004)
Rodriguez-Navarro, S. et al. Sus1, a functional component of the SAGA histone acetylase complex and the nuclear pore-associated mRNA export machinery. Cell 116, 75–86 (2004)
Cabal, G. G. et al. SAGA interacting factors confine sub-diffusion of transcribed genes to the nuclear envelope. Nature doi:10.1038/nature04752 (this issue)
Hediger, F., Neumann, F. R., Van Houwe, G., Dubrana, K. & Gasser, S. M. Live imaging of telomeres. yKu and Sir proteins define redundant telomere-anchoring pathways in yeast. Curr. Biol. 12, 2076–2089 (2002)
Taddei, A., Hediger, F., Neumann, F. R., Bauer, C. & Gasser, S. M. Separation of silencing from perinuclear anchoring functions in yeast Ku80, Sir4 and Esc1 proteins. EMBO J. 23, 1301–1312 (2004)
Gotta, M. et al. The clustering of telomeres and colocalization with Rap1, Sir3, and Sir4 proteins in wild-type Saccharomyces cerevisiae. J. Cell Biol. 134, 1349–1363 (1996)
Blobel, G. Gene gating: a hypothesis. Proc. Natl Acad. Sci. USA 82, 8527–8529 (1985)
Robinett, C. et al. In vivo localization of DNA sequences and visualization of large scale chromatin organization using lac-operator/repressor recognition. J. Cell Biol. 135, 1685–1700 (1996)
Heun, P., Laroche, T., Shimada, K., Furrer, P. & Gasser, S. M. Chromosome dynamics in the yeast interphase nucleus. Science 294, 2181–2186 (2001)
de la Cera, T., Herrero, P., Moreno-Herrero, F., Chaves, R. S. & Moreno, F. Mediator factor Med8p interacts with the hexokinase 2: implication in the glucose signalling pathway of Saccharomyces cerevisiae. J. Mol. Biol. 319, 703–714 (2002)
Rodriguez, A., de la Cera, T., Herrero, P. & Moreno, F. The hexokinase 2 protein regulates the expression of the GLK1, HXK1 and HXK2 genes of Saccharomyces cerevisiae. Biochem. J. 355, 625–631 (2001)
Sadowski, I., Ma, J., Triezenberg, S. & Ptashne, M. GAL4–VP16 is an unusually potent transcriptional activator. Nature 335, 563–564 (1988)
Tham, W. H., Wyithe, J. S., Ko Ferrigno, P., Silver, P. A. & Zakian, V. A. Localization of yeast telomeres to the nuclear periphery is separable from transcriptional repression and telomere stability functions. Mol. Cell 8, 189–199 (2001)
Hediger, F., Berthiau, A. S., van Houwe, G., Gilson, E. & Gasser, S. M. Subtelomeric factors antagonize telomere anchoring and Tel1-independent telomere length regulation. EMBO J. 25, 857–867 (2006)
Ishii, K., Arib, G., Lin, C., Van Houwe, G. & Laemmli, U. K. Chromatin boundaries in budding yeast: the nuclear pore connection. Cell 109, 551–562 (2002)
Menon, B. B. et al. Reverse recruitment: the Nup84 nuclear pore subcomplex mediates Rap1/Gcr1/Gcr2 transcriptional activation. Proc. Natl Acad. Sci. USA 102, 5749–5754 (2005)
Gill, G. & Ptashne, M. Negative effect of the transcriptional activator GAL4. Nature 334, 721–724 (1988)
Ito, T. et al. A comprehensive two-hybrid analysis to explore the yeast protein interactome. Proc. Natl Acad. Sci. USA 98, 4569–4574 (2001)
Gavin, A. C. et al. Functional organization of the yeast proteome by systematic analysis of protein complexes. Nature 415, 141–147 (2002)
O'Sullivan, J. M. et al. Gene loops juxtapose promoters and terminators in yeast. Nature Genet. 36, 1014–1018 (2004)
Hediger, F., Taddei, A., Neumann, F. R. & Gasser, S. M. Methods for visualizing chromatin dynamics in living yeast. Methods Enzymol. 375, 345–365 (2004)
Sage, D., Neumann, F. R., Hediger, F., Gasser, S. M. & Unser, M. Automatic tracking of individual fluorescence particles: application to the study of chromosome dynamics. IEEE Trans. Image Process. 14, 1372–1383 (2005)
Gartenberg, M. R., Neumann, F. R., Laroche, T., Blaszczyk, M. & Gasser, S. M. Sir-mediated repression can occur independently of chromosomal and subnuclear contexts. Cell 119, 955–967 (2004)
Vandesompele, J. et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3, research0034.1–research0034.11 (2002)
Schmid, M. et al. Nup-PI: The nucleopore-promoter interaction of genes in yeast. Mol Cell 21, 379–391 (2006)
Acknowledgements
We thank T. Laroche, the imaging platform of the FMI, and the genomics platform of the NCCR for assistance, and E. Heard, A. Peters, G. Almouzni, D. Schübeler and M. Gartenberg for helpful suggestions, as well as G. Cabal and U. Nehrbass for sharing unpublished results. Our research is supported by the Swiss National Science Foundation, the NCCR programme ‘Frontiers in Genetics’, and the Novartis Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
Supplementary information
Supplementary Notes
This file contains Supplementary Tables 1–3 and Supplementary Figure Legends. (DOC 70 kb)
Supplementary Figure 1
Abundance of the elongation-specific form of the RNA pol II on HXK1. (PDF 17 kb)
Supplementary Figure 2
HXK1 dynamics on glucose versus galactose media. (PDF 42 kb)
Supplementary Figure 3
Targeted VP16 allows variegated expression of subtelomeric ADE2 gene. (PDF 32 kb)
Supplementary Figure 4
VP16 targeting increases HXK1 dynamics. (PDF 35 kb)
Rights and permissions
About this article
Cite this article
Taddei, A., Van Houwe, G., Hediger, F. et al. Nuclear pore association confers optimal expression levels for an inducible yeast gene. Nature 441, 774–778 (2006). https://doi.org/10.1038/nature04845
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature04845
This article is cited by
-
Adaptive partitioning of a gene locus to the nuclear envelope in Saccharomyces cerevisiae is driven by polymer-polymer phase separation
Nature Communications (2023)
-
A R-loop sensing pathway mediates the relocation of transcribed genes to nuclear pore complexes
Nature Communications (2023)
-
Indel driven rapid evolution of core nuclear pore protein gene promoters
Scientific Reports (2023)
-
Engineering 3D genome organization
Nature Reviews Genetics (2021)
-
Coaching from the sidelines: the nuclear periphery in genome regulation
Nature Reviews Genetics (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.