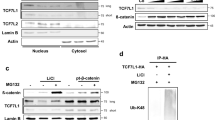
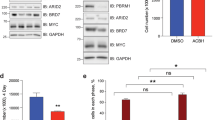
Abstract
Although constitutive activation of β-catenin/Tcf signalling is implicated in the development of human cancers1, the mechanisms by which the β-catenin/Tcf pathway promotes tumorigenesis are incompletely understood. Messenger RNA turnover has a major function in regulating gene expression and is responsive to developmental and environmental signals. mRNA decay rates are dictated by cis-acting elements within the mRNA and by trans-acting factors, such as RNA-binding proteins (reviewed in refs 2, 3). Here we show that β-catenin stabilizes the mRNA encoding the F-box protein βTrCP1, and identify the RNA-binding protein CRD-BP (coding region determinant-binding protein) as a previously unknown target of β-catenin/Tcf transcription factor. CRD-BP binds to the coding region of βTrCP1 mRNA. Overexpression of CRD-BP stabilizes βTrCP1 mRNA and elevates βTrCP1 levels (both in cells and in vivo), resulting in the activation of the Skp1-Cullin1-F-box protein (SCF)βTrCP E3 ubiquitin ligase and in accelerated turnover of its substrates including IκB and β-catenin. CRD-BP is essential for the induction of both βTrCP1 and c-Myc by β-catenin signalling in colorectal cancer cells. High levels of CRD-BP that are found in primary human colorectal tumours exhibiting active β-catenin/Tcf signalling implicates CRD-BP induction in the upregulation of βTrCP1, in the activation of dimeric transcription factor NF-κB and in the suppression of apoptosis in these cancers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Polakis, P. The oncogenic activation of β-catenin. Curr. Opin. Genet. Dev. 9, 15–21 (1999)
Guhaniyogi, J. & Brewer, G. Regulation of mRNA stability in mammalian cells. Gene 265, 11–23 (2001)
Wilusz, C. J., Wormington, M. & Peltz, S. W. The cap-to-tail guide to mRNA turnover. Nature Rev. Mol. Cell Biol. 2, 237–246 (2001)
Eastman, Q. & Grosschedl, R. Regulation of LEF-1/TCF transcription factors by Wnt and other signals. Curr. Opin. Cell Biol. 11, 233–240 (1999)
Spiegelman, V. S. et al. Wnt/β-catenin signaling induces the expression and activity of βTrCP ubiquitin ligase receptor. Mol. Cell 5, 877–882 (2000)
Nielsen, J. et al. A family of insulin-like growth factor II mRNA-binding proteins represses translation in late development. Mol. Cell. Biol. 19, 1262–1270 (1999)
Nielsen, F. C., Nielsen, J. & Christiansen, J. A family of IGF-II mRNA binding proteins (IMP) involved in RNA trafficking. Scand. J. Clin. Lab. Invest. Suppl. 234, 93–99 (2001)
Runge, S. et al. H19 RNA binds four molecules of insulin-like growth factor II mRNA-binding protein. J. Biol. Chem. 275, 29562–29569 (2000)
Atlas, R., Behar, L., Elliott, E. & Ginzburg, I. The insulin-like growth factor mRNA binding-protein IMP-1 and the Ras-regulatory protein G3BP associate with tau mRNA and HuD protein in differentiated P19 neuronal cells. J. Neurochem. 89, 613–626 (2004)
Bernstein, P. L., Herrick, D. J., Prokipcak, R. D. & Ross, J. Control of c-myc mRNA half-life in vitro by a protein capable of binding to a coding region stability determinant. Genes Dev. 6, 642–654 (1992)
Doyle, G. A. et al. The c-myc coding region determinant-binding protein: a member of a family of KH domain RNA-binding proteins. Nucleic Acids Res. 26, 5036–5044 (1998)
Prokipcak, R. D., Herrick, D. J. & Ross, J. Purification and properties of a protein that binds to the C-terminal coding region of human c-myc mRNA. J. Biol. Chem. 269, 9261–9269 (1994)
Tessier, C. R., Doyle, G. A., Clark, B. A., Pitot, H. C. & Ross, J. Mammary tumor induction in transgenic mice expressing an RNA-binding protein. Cancer Res. 64, 209–214 (2004)
Kudo, Y. et al. Role of F-box protein βTrcp1 in mammary gland development and tumorigenesis. Mol. Cell. Biol. 24, 8184–8194 (2004)
Fuchs, S. Y., Spiegelman, V. S. & Kumar, K. G. The many faces of β-TrCP E3 ubiquitin ligases: reflections in the magic mirror of cancer. Oncogene 23, 2028–2036 (2004)
Roose, J. et al. Synergy between tumor suppressor APC and the β-catenin-Tcf4 target Tcf1. Science 285, 1923–1926 (1999)
Morin, P. J. β-Catenin signaling and cancer. BioEssays 21, 1021–1030 (1999)
Ougolkov, A. et al. Associations among β-TrCP, an E3 ubiquitin ligase receptor, β-catenin, and NF-κB in colorectal cancer. J. Natl Cancer Inst. 96, 1161–1170 (2004)
Lin, A. & Karin, M. NF-κB in cancer: a marked target. Semin. Cancer Biol. 13, 107–114 (2003)
Ross, J., Lemm, I. & Berberet, B. Overexpression of an mRNA-binding protein in human colorectal cancer. Oncogene 20, 6544–6550 (2001)
He, T. C. et al. Identification of c-MYC as a target of the APC pathway. Science 281, 1509–1512 (1998)
Hansen, T. V. et al. Dwarfism and impaired gut development in insulin-like growth factor II mRNA-binding protein 1-deficient mice. Mol. Cell. Biol. 24, 4448–4464 (2004)
Leeds, P. et al. Developmental regulation of CRD-BP, an RNA-binding protein that stabilizes c-myc mRNA in vitro. Oncogene 14, 1279–1286 (1997)
Ioannidis, P. et al. CRD-BP: a c-Myc mRNA stabilizing protein with an oncofetal pattern of expression. Anticancer Res. 23, 2179–2183 (2003)
Ioannidis, P. et al. Expression of the RNA-binding protein CRD-BP in brain and non-small cell lung tumors. Cancer Lett. 209, 245–250 (2004)
Ioannidis, P. et al. C-MYC and IGF-II mRNA-binding protein (CRD-BP/IMP-1) in benign and malignant mesenchymal tumors. Int. J. Cancer 94, 480–484 (2001)
Ioannidis, P. et al. 8q24 Copy number gains and expression of the c-myc mRNA stabilizing protein CRD-BP in primary breast carcinomas. Int. J. Cancer 104, 54–59 (2003)
Ross, J. Messenger RNA turnover in cell-free extracts from higher eukaryotes. Methods Mol. Biol. 118, 459–476 (1999)
Acknowledgements
We thank K. Spiegelman for help with the manuscript preparation. This work was supported by an American Cancer Society Award (to V.S.S.). The work was supported in part by a University of Pennsylvania Cancer Center Pilot Grant and an NCI grant (to S.Y.F.), by NIH grants (to J.R.) and by the Japanese Ministry of Education, Science, Sports, Technology and Culture, by the Ministry of Health, Labor and Welfare, and by the Japan Society for the Promotion of Science (to T.M.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
Supplementary information
Supplementary Figure 1
This figure provides details on interaction of CRD–BP with the mRNA of β-TrCP1. It also shows that overexpression of CRD-BP in cells led to accumulation of its steady state levels. (PDF 239 kb)
Supplementary Figure 2
This figure shows that CRD-BP expression does not affect IκB phosphorylation. It also contains detailed characterization of CRD–BP shRNA used in this study, and shows that knockdown of endogenous CRD–BP by specific shRNA prevents β-catenin/Tcf-dependent stabilization of endogenous β-TrCP1 mRNA. (PDF 371 kb)
Supplementary Figure 3
This figure demonstrates the transient nature of Wnt3A-induced β-catenin-DNA interaction that can be significantly prolonged by CRD-BP knockdown. It also shows that knock down of CRD–BP noticeably decreased βTrCP1 expression, and leads to the inhibition of NF-κB activity, induction of apoptosis, and suppression of colony formation in colorectal cancer cells . (PDF 374 kb)
Supplementary Methods
This section provides detailed methods used in this manuscript. (PDF 164 kb)
Rights and permissions
About this article
Cite this article
Noubissi, F., Elcheva, I., Bhatia, N. et al. CRD-BP mediates stabilization of βTrCP1 and c-myc mRNA in response to β-catenin signalling. Nature 441, 898–901 (2006). https://doi.org/10.1038/nature04839
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature04839
This article is cited by
-
Ischemia-reperfusion injury: molecular mechanisms and therapeutic targets
Signal Transduction and Targeted Therapy (2024)
-
Elevated expression of the RNA-binding protein IGF2BP1 enhances the mRNA stability of INHBA to promote the invasion and migration of esophageal squamous cancer cells
Experimental Hematology & Oncology (2023)
-
IGF2BP1 regulates the cargo of extracellular vesicles and promotes neuroblastoma metastasis
Oncogene (2023)
-
CircPTPRA blocks the recognition of RNA N6-methyladenosine through interacting with IGF2BP1 to suppress bladder cancer progression
Molecular Cancer (2021)
-
LncRNA LINRIS stabilizes IGF2BP2 and promotes the aerobic glycolysis in colorectal cancer
Molecular Cancer (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.