Abstract
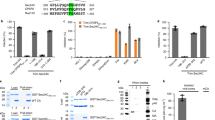
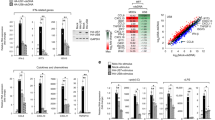
Human cytomegalovirus (HCMV) prevents the display of class I major histocompatibility complex (MHC) peptide complexes at the surface of infected cells as a means of escaping immune detection1. Two HCMV-encoded immunoevasins, US2 and US11, induce the dislocation of class I MHC heavy chains from the endoplasmic reticulum membrane and target them for proteasomal degradation in the cytosol2,3. Although the outcome of the dislocation reactions catalysed is similar, US2 and US11 operate differently: Derlin-1 is a key component of the US11 but not the US2 pathway4. So far, proteins essential for US2-dependent dislocation have not been identified. Here we compare interacting partners of wild-type US2 with those of a dislocation-incompetent US2 mutant, and identify signal peptide peptidase (SPP) as a partner for the active form of US2. We show that a decrease in SPP levels by RNA-mediated interference inhibits heavy-chain dislocation by US2 but not by US11. Our data implicate SPP in the US2 pathway and indicate the possibility of a previously unknown function for this intramembrane-cleaving aspartic protease in dislocation from the endoplasmic reticulum.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Tortorella, D., Gewurz, B. E., Furman, M. H., Schust, D. J. & Ploegh, H. L. Viral subversion of the immune system. Annu. Rev. Immunol. 18, 861–926 (2000)
Wiertz, E. J. et al. Sec61-mediated transfer of a membrane protein from the endoplasmic reticulum to the proteasome for destruction. Nature 384, 432–438 (1996)
Wiertz, E. J. et al. The human cytomegalovirus US11 gene product dislocates MHC class I heavy chains from the endoplasmic reticulum to the cytosol. Cell 84, 769–779 (1996)
Lilley, B. N. & Ploegh, H. L. A membrane protein required for dislocation of misfolded proteins from the ER. Nature 429, 834–840 (2004)
Ellgaard, L. & Helenius, A. Quality control in the endoplasmic reticulum. Nature Rev. Mol. Cell Biol. 4, 181–191 (2003)
Meusser, B., Hirsch, C., Jarosch, E. & Sommer, T. ERAD: the long road to destruction. Nature Cell Biol. 7, 766–772 (2005)
Gewurz, B. E., Wang, E. W., Tortorella, D., Schust, D. J. & Ploegh, H. L. Human cytomegalovirus US2 endoplasmic reticulum-lumenal domain dictates association with major histocompatibility complex class I in a locus-specific manner. J. Virol. 75, 5197–5204 (2001)
Lilley, B. N. & Ploegh, H. L. Multiprotein complexes that link dislocation, ubiquitination, and extraction of misfolded proteins from the endoplasmic reticulum membrane. Proc. Natl Acad. Sci. USA 102, 14296–14301 (2005)
Ye, Y., Meyer, H. H. & Rapoport, T. A. The AAA ATPase Cdc48/p97 and its partners transport proteins from the ER into the cytosol. Nature 414, 652–656 (2001)
Ye, Y., Shibata, Y., Yun, C., Ron, D. & Rapoport, T. A. A membrane protein complex mediates retro-translocation from the ER lumen into the cytosol. Nature 429, 841–847 (2004)
Gewurz, B. E., Ploegh, H. L. & Tortorella, D. US2, a human cytomegalovirus-encoded type I membrane protein, contains a non-cleavable amino-terminal signal peptide. J. Biol. Chem. 277, 11306–11313 (2002)
Furman, M. H., Ploegh, H. L. & Tortorella, D. Membrane-specific, host-derived factors are required for US2- and US11-mediated degradation of major histocompatibility complex class I molecules. J. Biol. Chem. 277, 3258–3267 (2002)
Friedmann, E. et al. Consensus analysis of signal peptide peptidase and homologous human aspartic proteases reveals opposite topology of catalytic domains compared with presenilins. J. Biol. Chem. 279, 50790–50798 (2004)
Weihofen, A., Binns, K., Lemberg, M. K., Ashman, K. & Martoglio, B. Identification of signal peptide peptidase, a presenilin-type aspartic protease. Science 296, 2215–2218 (2002)
Martoglio, B. & Golde, T. E. Intramembrane-cleaving aspartic proteases and disease: presenilins, signal peptide peptidase and their homologs. Hum. Mol. Genet. 12 (spec. no. 2), R201–R206 (2003)
Lemberg, M. K., Bland, F. A., Weihofen, A., Braud, V. M. & Martoglio, B. Intramembrane proteolysis of signal peptides: an essential step in the generation of HLA-E epitopes. J. Immunol. 167, 6441–6446 (2001)
McLauchlan, J., Lemberg, M. K., Hope, G. & Martoglio, B. Intramembrane proteolysis promotes trafficking of hepatitis C virus core protein to lipid droplets. EMBO J. 21, 3980–3988 (2002)
Martoglio, B., Graf, R. & Dobberstein, B. Signal peptide fragments of preprolactin and HIV-1 p-gp160 interact with calmodulin. EMBO J. 16, 6636–6645 (1997)
Crawshaw, S. G., Martoglio, B., Meacock, S. L. & High, S. A misassembled transmembrane domain of a polytopic protein associates with signal peptide peptidase. Biochem. J. 384, 9–17 (2004)
Nyborg, A. C. et al. Signal peptide peptidase forms a homodimer that is labeled by an active site-directed gamma-secretase inhibitor. J. Biol. Chem. 279, 15153–15160 (2004)
Brummelkamp, T. R., Bernards, R. & Agami, R. A system for stable expression of short interfering RNAs in mammalian cells. Science 296, 550–553 (2002)
Martoglio, B. Intramembrane proteolysis and post-targeting functions of signal peptides. Biochem. Soc. Trans. 31, 1243–1247 (2003)
Roques, B. P., Lucas-Soroca, E., Chaillet, P., Costentin, J. & Fournie-Zaluski, M. C. Complete differentiation between enkephalinase and angiotensin-converting enzyme inhibition by retro-thiorphan. Proc. Natl Acad. Sci. USA 80, 3178–3182 (1983)
Tarasova, N. I. et al. Transmembrane inhibitors of P-glycoprotein, an ABC transporter. J. Med. Chem. 48, 3768–3775 (2005)
Kornilova, A. Y., Das, C. & Wolfe, M. S. Differential effects of inhibitors on the gamma-secretase complex. Mechanistic implications. J. Biol. Chem. 278, 16470–16473 (2003)
Lemberg, M. K. & Martoglio, B. On the mechanism of SPP-catalysed intramembrane proteolysis; conformational control of peptide bond hydrolysis in the plane of the membrane. FEBS Lett. 564, 213–218 (2004)
Blom, D., Hirsch, C., Stern, P., Tortorella, D. & Ploegh, H. L. A glycosylated type I membrane protein becomes cytosolic when peptide: N-glycanase is compromised. EMBO J. 23, 650–658 (2004)
Misaghi, S., Pacold, M. E., Blom, D., Ploegh, H. L. & Korbel, G. A. Using a small molecule inhibitor of peptide: N-glycanase to probe its role in glycoprotein turnover. Chem. Biol. 11, 1677–1687 (2004)
Shaffer, A. L. et al. XBP1, downstream of Blimp-1, expands the secretory apparatus and other organelles, and increases protein synthesis in plasma cell differentiation. Immunity 21, 81–93 (2004)
Brown, M. S., Ye, J., Rawson, R. B. & Goldstein, J. L. Regulated intramembrane proteolysis: a control mechanism conserved from bacteria to humans. Cell 100, 391–398 (2000)
Acknowledgements
We thank S. High for reagents, A. Weihofen and M. Wolfe for technical advice and insight, A. Weihofen and M. Lemberg for critical reading of the manuscript, T. DiCesare for Fig. 4c, and members of the Ploegh laboratory for discussions. J.L. is a Gulbenkian PhD Program Fellow with a grant from the Portuguese Science and Technology Foundation (FCT). B.N.L. was supported by a Howard Hughes Medical Institute Predoctoral Fellowship. This work was supported by grants from the NIH to H.L.P. Author Contributions B.N.L. designed the HA-US2 and HA-US2186 constructs, supplied the retroviral supernatants for transduction of astrocytoma cells and provided critical reading of the manuscript. V.N. and D.T. designed and supplied the untagged US2-CD4-US2 cDNA. All other constructs were designed by J.L. Mass spectrometry analysis was performed by E.S. All experiments were designed and performed by J.L. Data analysis, interpretation and writing of the manuscript were by J.L. and H.L.P.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
Supplementary information
Supplementary Figure 1
Both Kbss-HA-TEV-tagged US2 constructs are targeted to the ER membrane and associate with heavy chains. (JPG 273 kb)
Supplementary Figure 2
Large-scale affinity purification of US2 and US2-associated proteins from U373-MG control cells (-) or cells expressing HA-TEV-US2 or HA-TEV-US2186. (JPG 338 kb)
Supplementary Figure 3
Mismatch of the SPP.1 shRNA sequence ablates its SPP knockdown effect and confirms RNAi specificity. (JPG 152 kb)
Supplementary Figure 4
Aminoacid sequences of wild type untagged US2 and all HA-tagged US2 constructs used in these studies. (JPG 788 kb)
Supplementary Figure 5
The US2 TMD is dispensable for association with HC. (JPG 257 kb)
Supplementary Figure 6
Both monomeric and dimeric forms of SPP are observed in association with dislocation-competent US2; however the ratio of SDS-stable SPP dimer/monomer is dependent on the treatment of samples before loading on the gel. (JPG 330 kb)
Supplementary Figure Legends
This file contains text to accompany the above Supplementary Figures. (DOC 37 kb)
Rights and permissions
About this article
Cite this article
Loureiro, J., Lilley, B., Spooner, E. et al. Signal peptide peptidase is required for dislocation from the endoplasmic reticulum. Nature 441, 894–897 (2006). https://doi.org/10.1038/nature04830
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature04830
This article is cited by
-
Mechanisms of substrate processing during ER-associated protein degradation
Nature Reviews Molecular Cell Biology (2023)
-
A signal peptide peptidase is required for ER-symbiosome proximal association and protein secretion
Nature Communications (2023)
-
Identification of HM13 as a prognostic indicator and a predictive biomarker for immunotherapy in hepatocellular carcinoma
BMC Cancer (2022)
-
Spatially Resolved Tagging of Proteolytic Neo-N termini with Subtiligase-TM
The Journal of Membrane Biology (2021)
-
Signal peptide peptidase promotes tumor progression via facilitating FKBP8 degradation
Oncogene (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.