Abstract
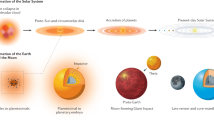
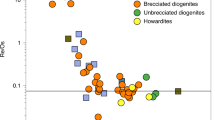
The Earth took 30–40 million years to accrete from smaller ‘planetesimals’. Many of these planetesimals had metallic iron cores and during growth of the Earth this metal re-equilibrated with the Earth's silicate mantle, extracting siderophile (‘iron-loving’) elements into the Earth's iron-rich core. The current composition of the mantle indicates that much of the re-equilibration took place in a deep (> 400 km) molten silicate layer, or ‘magma ocean’, and that conditions became more oxidizing with time as the Earth grew. The high-pressure nature of the core-forming process led to the Earth's core being richer in low-atomic-number elements, notably silicon and possibly oxygen, than the cores of the smaller planetesimal building blocks.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Chambers, J. E. Planetary accretion in the inner solar system. Earth Planet. Sci. Lett. 224, 241–252 (2004)
Kleine, T., Munker, C., Mezger, K. & Palme, H. Rapid accretion and early core formation on asteroids and the terrestrial planets from Hf–W chronometry. Nature 418, 952–955 (2002)
McDonough, W. F. & Sun, S.-s. The composition of the Earth. Chem. Geol. 120, 223–253 (1995)
Li, J. & Agee, C. B. Geochemistry of mantle-core differentiation at high pressure. Nature 381, 686–689 (1996)
Righter, K. & Drake, M. J. Metal/silicate equilibrium in the early Earth–New constraints from the volatile moderately siderophile elements Ga, Cu, P, and Sn. Geochim. Cosmochim. Acta 64, 3581–3597 (2000)
Wade, J. & Wood, B. J. Core formation and the oxidation state of the Earth. Earth Planet. Sci. Lett. 236, 78–95 (2005)
Frost, D. J. et al. Experimental evidence for the existence of iron-rich metal in the Earth's lower mantle. Nature 428, 409–412 (2004)
O'Neill, H. S. C. The origin of the Moon and the early history of the Earth—A chemical model. 2. The Earth. Geochim. Cosmochim. Acta 55, 1159–1172 (1991)
Wood, B. J. & Halliday, A. N. Cooling of the earth and core formation after the giant impact. Nature 437, 1345–1348 (2005)
Youdin, A. N. & Shu, F. H. Planetesimal formation by gravitational instability. Astrophys. J. 580, 494–505 (2002)
Poppe, T., Blum, J. & Henning, T. Analogous experiments on the stickiness of micron sized planetary dust. Astrophys. J. 533, 454–471 (2000)
Weidenschilling, S. J. Aerodynamics of solid bodies in the solar nebula. Mon. Not. R. Astron. Soc. 180, 57–70 (1977)
Benz, W. & Asphaug, E. Catastrophic disruptions revisited. Icarus 142, 5–20 (1999)
Leinhardt, Z. M., Richardson, D. C. & Quinn, T. Direct N-body simulations of rubble-pile collisions. Icarus 146, 133–151 (2000)
Wetherill, G. W. & Stewart, G. R. Formation of planetary embryos: effects of fragmentation, low relative velocity, and independent variation of eccentricity and inclination. Icarus 106, 190–209 (1993)
Kokubo, E. & Ida, S. Oligarchic growth of protoplanets. Icarus 131, 171–178 (1998)
Weidenschilling, S. J., Spaute, D., Davis, D. R., Marzari, F. & Ohtsuki, K. Accretional evolution of a planetesimal swarm. 2. The terrestrial zone. Icarus 128, 429–455 (1997)
Taylor, S. R. & Norman, S. R. in Origin of the Earth (eds Newsom, H. E. & Jones, J. H.) 29–44 (Oxford Univ. Press, New York, 1990)
Yin, Q. Z. et al. A short timescale for terrestrial planet formation from Hf-W chronometry of meteorites. Nature 418, 949–952 (2002)
Stevenson, D. J. in Origin of the Earth (eds Newsom, H. E. & Drake, J. H.) 231–249 (Oxford Univ. Press, New York, 1990)
Yoshino, T., Walter, M. J. & Katsura, T. Core formation in planetesimals triggered by permeable flow. Nature 422, 154–157 (2003)
Ghosh, A. & McSween, H. Y. A thermal model for the differentiation of Asteroid 4 Vesta. Icarus 134, 187–206 (1998)
Shukolyukov, A. & Lugmair, G. W. Live iron-60 in the early solar system. Science 259, 1138–1142 (1993)
Kunihiro, T., Rubin, A. E., McKeegan, K. D. & Wasson, J. T. Initial 26Al/27Al in carbonaceous-chondrite chondrules: Too little 26Al to melt asteroids. Geochim. Cosmochim. Acta 68, 2947–2957 (2004)
Chambers, J. E. & Wetherill, G. W. Making the terrestrial planets: N-body integrations of planetary embryos in three dimensions. Icarus 136, 304–327 (1998)
Allegre, C. J., Poirier, J.-P., Humler, E. & Hofmann, A. W. The chemical composition of the Earth. Earth Planet. Sci. Lett. 134, 515–526 (1995)
Wasson, J. T. Meteorites: Their Record of Early Solar-System History (W.H. Freeman, New York, 1985)
Drake, M. J. & Righter, K. Determining the composition of the Earth. Nature 416, 33–39 (2002)
McDonough, W. F. in The Mantle and Core (ed. Carlson, R. W.) 547–568 (Elsevier-Pergamon, Oxford, 2003)
Newsom, H. E. in Origin of the Earth (eds Newsom, H. E. & Jones, J. H.) 273–288 (Oxford Univ. Press, New York, 1990)
O'Neill, H. S. C. & Palme, H. in The Earth's Mantle–Composition, Structure and Evolution (ed. Jackson, I.) 3–127 (Cambridge Univ. Press, Cambridge, UK, 1998)
Ringwood, A. E. Chemical evolution of the terrestrial planets. Geochim. Cosmochim. Acta 30, 41–104 (1966)
Harper, C. L. Jr & Jacobsen, S. B. Evidence for 182Hf in the early Solar System and constraints on the timescale of terrestrial accretion and core formation. Geochim. Cosmochim. Acta 60, 1131–1153 (1996)
Arculus, R. J. & Delano, J. W. Siderophile element abundances in the upper mantle: Evidence for a sulfide signature and equilibrium with the core. Geochim. Cosmochim. Acta 45, 1331–1343 (1981)
Jones, J. H. & Drake, M. J. Geochemical constraints on core formation in the Earth. Nature 322, 221–228 (1986)
Chou, C. L. Fractionation of siderophile elements in the Earth's upper mantle. Proc. Ninth Lunar Sci. Conf., 219–230 (1978)
Wänke, H. Constitution of the terrestrial planets. Phil. Trans. R. Soc. Lond. A 303, 287–302 (1981)
Thibault, Y. & Walter, M. J. The influence of pressure and temperature on the metal-silicate partition coefficients of nickel and cobalt in a model C1 chondrite and implications for metal segregation in a deep magma ocean. Geochim. Cosmochim. Acta 59, 991–1002 (1995)
Righter, K., Drake, M. J. & Yaxley, G. Prediction of siderophile element metal-silicate partition coefficients to 20 GPa and 2800 degrees C: The effects of pressure, temperature, oxygen fugacity, and silicate and metallic melt compositions. Phys. Earth Planet. Inter. 100, 115–134 (1997)
Li, J. & Agee, C. B. The effect of pressure, temperature, oxygen fugacity and composition on partitioning of nickel and cobalt between liquid Fe-Ni-S alloy and liquid silicate: Implications for the Earth's core formation. Geochim. Cosmochim. Acta 65, 1821–1832 (2001)
Gessmann, C. K. & Rubie, D. C. The effect of temperature on the partitioning of nickel, cobalt, manganese, chromium, and vanadium at 9 GPa and constraints on formation of the Earth's core. Geochim. Cosmochim. Acta 62, 867–882 (1998)
Righter, K. & Drake, M. J. Metal-silicate equilibrium in a homogeneously accreting earth: New results for Re. Earth Planet. Sci. Lett. 146, 541–553 (1997)
Righter, K. & Drake, M. J. Effect of water on metal-silicate partitioning of siderophile elements: a high pressure and temperature terrestrial magma ocean and core formation. Earth Planet. Sci. Lett. 171, 383–399 (1999)
Rubie, D. C., Melosh, H. J., Reid, J. E., Liebske, C. & Righter, K. Mechanisms of metal-silicate equilibration in the terrestrial magma ocean. Earth Planet. Sci. Lett. 205, 239–255 (2003)
Stevenson, D. J. Models of the Earth's Core. Science 214, 611–619 (1981)
Cameron, A. G. W. & Benz, W. Origin of the Moon and the single impact hypothesis IV. Icarus 92, 204–216 (1991)
Canup, R. M. & Asphaug, E. Origin of the Moon in a giant impact near the end of the Earth's formation. Nature 412, 708–712 (2001)
Karato, S.-i. & Rama Murthy, V. Core formation and chemical equilibrium in the Earth–I. Physical considerations. Phys. Earth Planet. Inter. 100, 61–79 (1997)
Abe, Y. Thermal and chemical evolution of the terrestrial magma ocean. Phys. Earth Planet. Inter. 100, 27–39 (1997)
Solomatov, V. S. in Origin of the Earth and Moon (eds Canup, R. M. & Righter, K.) 323–360 (Univ. Arizona Press, Tucson, 2000)
Chabot, N. L. & Agee, C. B. Core formation in the Earth and Moon: new experimental constraints from V, Cr, and Mn. Geochim. Cosmochim. Acta 67, 2077–2091 (2003)
Chabot, N. L., Draper, D. S. & Agee, C. B. Conditions of core formation in the Earth: Constraints from nickel and cobalt partitioning. Geochim. Cosmochim. Acta 69, 2141–2151 (2005)
Cottrell, E. & Walker, D. Constraints on core formation from Pt partitioning in mafic silicate liquids at high temperatures. Geochim. Cosmochim. Acta (in the press)
Wood, B. J., Bryndzia, L. T. & Johnson, K. E. Mantle oxidation state and its relationship to tectonic environment and fluid speciation. Science 248, 337–345 (1990)
Hunten, D. M. Atmospheric evolution of the terrestrial planets. Science 259, 915–920 (1993)
Herd, C. D. K., Borg, L. E., Jones, J. H. & Papike, J. J. Oxygen fugacity and geochemical variations in the martian basalts: Implications for martian basalt petrogenesis and the oxidation state of the upper mantle of Mars. Geochim. Cosmochim. Acta 66, 2025–2036 (2002)
Wood, B. J. Phase transformations and partitioning relations in peridotite under lower mantle conditions. Earth Planet. Sci. Lett. 174, 341–354 (2000)
McCammon, C. Perovskite as a possible sink for ferric iron in the lower mantle. Nature 387, 694–696 (1997)
Wood, B. J. & Rubie, D. C. The effect of alumina on phase transformations at the 660-kilometer discontinuity from Fe-Mg partitioning experiments. Science 273, 1522–1524 (1996)
Canup, R. M. Simulations of a late lunar-forming impact. Icarus 168, 433–456 (2004)
Kleine, T., Palme, H., Mezger, K. & Halliday, A. N. Hf-W chronometry of lunar metals and the age and early differentiation of the Moon. Science 310, 1671–1674 (2005)
Ohtani, E., Yurimoto, H. & Seto, S. Element partitioning between metallic liquid, silicate liquid, and lower-mantle minerals: implications for core formation of the Earth. Phys. Earth Planet. Inter. 100, 97–114 (1997)
Francis, R. D. Sulfide globules in mid-ocean ridge basalts (MORB), and the effect of oxygen abundance in Fe-S-O liquids on the ability of those liquids to partition metals from MORB and komatiite magmas. Chem. Geol. 85, 199–213 (1990)
Jones, J. H., Hart, S. R. & Benjamin, T. M. Experimental partitioning near the Fe-FeS eutectic, with an emphasis on elements important to iron meteorite chronologies (Pb, Ag, Pd, and Tl). Geochim. Cosmochim. Acta 57, 453–460 (1993)
Birch, F. Elasticity and constitution of the Earth's interior. J. Geophys. Res. 57, 227–286 (1952)
Newsom, H. E. in Global Earth Physics: a Handbook of Physical Constants (ed. Ahrens, T. J.) 159–189 (American Geophysical Union, Washingon DC, 1995)
Anderson, O. L. & Isaak, D. G. Another look at the core density deficit of Earth's outer core. Phys. Earth Planet. Inter. 131, 19–27 (2002)
Poirier, J. P. Light elements in the Earth's outer core—a critical-review. Phys. Earth Planet. Inter. 85, 319–337 (1994)
Dreibus, G. & Palme, H. Cosmochemical constraints on the sulfur content in the Earth's core. Geochim. Cosmochim. Acta 60, 1125–1130 (1996)
Kilburn, M. R. & Wood, B. J. Metal-silicate partitioning and the incompatibility of S and Si during core formation. Earth Planet. Sci. Lett. 152, 139–148 (1997)
Gessmann, C. K., Wood, B. J., Rubie, D. C. & Kilburn, M. R. Solubility of silicon in liquid metal at high pressure: implications for the composition of the Earth's core. Earth Planet. Sci. Lett. 184, 367–376 (2001)
Ohtani, E. & Ringwood, A. E. Composition of the core. I. Solubility of oxygen in molten iron at high temperatures. Earth Planet. Sci. Lett. 71, 85–93 (1984)
Rubie, D. C., Gessmann, C. K. & Frost, D. J. Partitioning of oxygen during core formation on the Earth and Mars. Nature 429, 58–61 (2004)
Ohtani, E., Ringwood, A. E. & Hibberson, W. Composition of the core. II. Effect of high pressure on solubility of FeO in molten iron. Earth Planet. Sci. Lett. 71, 94–103 (1984)
O'Neill, H. S., Canil, D. & Rubie, D. C. Oxide-metal equilibria to 2500 degrees C and 25 GPa: Implications for core formation and the light component in the Earth's core. J. Geophys. Res. 103, 12239–12260 (1998)
Takafuji, N., Hirose, K., Mitome, N. & Bando, Y. Solubilities of O and Si in liquid iron in equilibrium with (Mg,Fe)SiO3 perovskite and the light elements in the core. Geophys. Res. Lett. 32, L06313 (2005)
Tronnes, R. G. & Frost, D. J. Peridotite melting and mineral-melt partitioning of major and minor elements at 22–24.5 GPa. Earth Planet. Sci. Lett. 197, 117–131 (2002)
Kilinc, A., Carmichael, I. S. E., Rivers, M. L. & Sack, R. O. The ferric-ferrous ratio of natural silicate liquids equilibrated in air. Contrib. Mineral. Petrol. 83, 136–140 (1983)
Acknowledgements
The constructive reviews of C. Agee and K. Righter are acknowledged with thanks. B.J.W. acknowledges the award of an ARC Federation Fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Wood, B., Walter, M. & Wade, J. Accretion of the Earth and segregation of its core. Nature 441, 825–833 (2006). https://doi.org/10.1038/nature04763
Issue Date:
DOI: https://doi.org/10.1038/nature04763
This article is cited by
-
Tetracarbonates in silicate melts may be at the origin of a deep carbon reservoir in the deep Earth
Communications Earth & Environment (2023)
-
Magma Ocean, Water, and the Early Atmosphere of Venus
Space Science Reviews (2023)
-
The Geochemical Legacy of Low-Temperature, Percolation-Driven Core Formation in Planetesimals
Earth, Moon, and Planets (2023)
-
Transient variation in seismic wave speed points to fast fluid movement in the Earth's outer core
Communications Earth & Environment (2022)
-
Oxygen isotope (δ18O, Δ′17O) insights into continental mantle evolution since the Archean
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.