Abstract
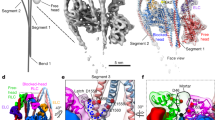
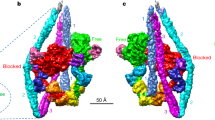
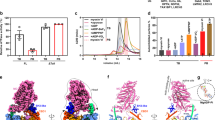
Unconventional myosin V (myoV) is an actin-based molecular motor that has a key function in organelle and mRNA transport, as well as in membrane trafficking1. MyoV was the first member of the myosin superfamily shown to be processive, meaning that a single motor protein can ‘walk’ hand-over-hand along an actin filament for many steps before detaching2,3,4. Full-length myoV has a low actin-activated MgATPase activity at low [Ca2+], whereas expressed constructs lacking the cargo-binding domain have a high activity regardless of [Ca2+] (refs 5–7). Hydrodynamic data and electron micrographs indicate that the active state is extended, whereas the inactive state is compact8,9,10. Here we show the first three-dimensional structure of the myoV inactive state. Each myoV molecule consists of two heads that contain an amino-terminal motor domain followed by a lever arm that binds six calmodulins. The heads are followed by a coiled-coil dimerization domain (S2) and a carboxy-terminal globular cargo-binding domain. In the inactive structure, bending of myoV at the head–S2 junction places the cargo-binding domain near the motor domain's ATP-binding pocket, indicating that ATPase inhibition might occur through decreased rates of nucleotide exchange. The actin-binding interfaces are unobstructed, and the lever arm is oriented in a position typical of strong actin-binding states. This structure indicates that motor recycling after cargo delivery might occur through transport on actively treadmilling actin filaments rather than by diffusion.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Reck-Peterson, S. L., Provance, D. W. Jr, Mooseker, M. S. & Mercer, J. A. Class V myosins. Biochim. Biophys. Acta 1496, 36–51 (2000)
Mehta, A. D. et al. Myosin-V is a processive actin-based motor. Nature 400, 590–593 (1999)
Forkey, J. N., Quinlan, M. E., Shaw, M. A., Corrie, J. E. & Goldman, Y. E. Three-dimensional structural dynamics of myosin V by single-molecule fluorescence polarization. Nature 422, 399–404 (2003)
Yildiz, A. et al. Myosin V walks hand-over-hand: single fluorophore imaging with 1.5-nm localization. Science 300, 2061–2065 (2003)
Wang, F. et al. Effect of ADP and ionic strength on the kinetic and motile properties of recombinant mouse myosin V. J. Biol. Chem. 275, 4329–4335 (2000)
Homma, K., Saito, J., Ikebe, R. & Ikebe, M. Ca2+-dependent regulation of the motor activity of myosin V. J. Biol. Chem. 275, 34766–34771 (2000)
Trybus, K. M., Krementsova, E. & Freyzon, Y. Kinetic characterization of a monomeric unconventional myosin V construct. J. Biol. Chem. 274, 27448–27456 (1999)
Wang, F. et al. Regulated conformation of myosin V. J. Biol. Chem. 279, 2333–2336 (2004)
Krementsov, D. N., Krementsova, E. B. & Trybus, K. M. Myosin V: Regulation by calcium, calmodulin, and the tail domain. J. Cell Biol. 164, 877–886 (2004)
Li, X. D., Mabuchi, K., Ikebe, R. & Ikebe, M. Ca2+-induced activation of ATPase activity of myosin Va is accompanied with a large conformational change. Biochem. Biophys. Res. Commun. 315, 538–545 (2004)
Frank, J. Three-Dimensional Electron Microscopy of Macromolecular Assemblies (Academic, San Diego, CA, 1996)
Terrak, M., Wu, G., Stafford, W. F., Lu, R. C. & Dominguez, R. Two distinct myosin light chain structures are induced by specific variations within the bound IQ motifs-functional implications. EMBO J. 22, 362–371 (2003)
Coureux, P. D., Sweeney, H. L. & Houdusse, A. Three myosin V structures delineate essential features of chemo-mechanical transduction. EMBO J. 23, 4527–4537 (2004)
Tama, F., Miyashita, O. & Brooks, C. L. Normal mode based flexible fitting of high-resolution structure into low-resolution experimental data from cryo-EM. J. Struct. Biol. 147, 315–326 (2004)
Cheney, R. E. et al. Brain myosin-V is a two-headed unconventional myosin with motor activity. Cell 75, 13–23 (1993)
Sweeney, H. L. et al. Kinetic tuning of myosin via a flexible loop adjacent to the nucleotide binding pocket. J. Biol. Chem. 273, 6262–6270 (1998)
Sellers, J. R. Regulation of cytoplasmic and smooth muscle myosin. Curr. Opin. Cell Biol. 3, 98–104 (1991)
Hackney, D. D., Levitt, J. D. & Suhan, J. Kinesin undergoes a 9 S to 6 S conformational transition. J. Biol. Chem. 267, 8696–8701 (1992)
Wendt, T., Taylor, D., Trybus, K. M. & Taylor, K. Three-dimensional image reconstruction of dephosphorylated smooth muscle heavy meromyosin reveals asymmetry in the interaction between myosin heads and placement of subfragment 2. Proc. Natl Acad. Sci. USA 98, 4361–4366 (2001)
Stock, M. F. et al. Formation of the compact confomer of kinesin requires a COOH-terminal heavy chain domain and inhibits microtubule-stimulated ATPase activity. J. Biol. Chem. 274, 14617–14623 (1999)
Coy, D. L., Hancock, W. O., Wagenbach, M. & Howard, J. Kinesin's tail domain is an inhibitory regulator of the motor domain. Nature Cell Biol. 1, 288–292 (1999)
Hackney, D. D. & Stock, M. F. Kinesin's IAK tail domain inhibits initial microtubule-stimulated ADP release. Nature Cell Biol. 2, 257–260 (2000)
Wang, F. S., Wolenski, J. S., Cheney, R. E., Mooseker, M. S. & Jay, D. G. Function of myosin-V in filopodial extension of neuronal growth cones. Science 273, 660–663 (1996)
Espreafico, E. M. et al. Primary structure and cellular localization of chicken brain myosin-V (p190), an unconventional myosin with calmodulin light chains. J. Cell Biol. 119, 1541–1557 (1992)
Star, E. N., Kwiatkowski, D. J. & Murthy, V. N. Rapid turnover of actin in dendritic spines and its regulation by activity. Nature Neurosci. 5, 239–246 (2002)
Forscher, P. & Smith, S. J. Actions of cytochalasins on the organization of actin filaments and microtubules in a neuronal growth cone. J. Cell Biol. 107, 1505–1516 (1988)
Ludtke, S. J., Baldwin, P. R. & Chiu, W. EMAN: semiautomated software for high-resolution single-particle reconstructions. J. Struct. Biol. 128, 82–97 (1999)
Winkler, H. & Taylor, K. A. Accurate marker-free alignment with simultaneous geometry determination and reconstruction of tilt series in electron tomography. Ultramicroscopy 106, 240–254 (2006)
Winkler, H. & Taylor, K. A. Focus gradient correction applied to tilt series image data used in electron tomography. J. Struct. Biol. 143, 24–32 (2003)
Rayment, I. et al. Structure of the actin–myosin complex and its implications for muscle contraction. Science 261, 58–65 (1993)
Acknowledgements
We thank H. Winkler for his guidance in focus gradient correction and 3D volume classification. We also thank the National Institutes of Health Research Resource for MMTSB and C. L. Brooks 3rd for making NMFF available. This research was supported by grants from the National Institutes of Health (to K.M.T. and K.A.T.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The atomic coordinates of the final myoV atomic model have been deposited in the Protein Data Bank with accession number 2DFS. The density volume for the flower motif shown in Fig. 2 has been deposited in the European Bioinformatics Institute under accession code EMD-1201. Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
Supplementary information
Supplementary Notes
This file contains Supplementary Movie Legends and Supplementary Figure Legends. This file also contains Supplementary Methods (model fitting, Electron micrographs of actin decorated with myosin V in the inhibited conformation and a description of the conditions used to make the specimens of myoV decorated actin. (PDF 689 kb)
Supplementary Figures
Contains a slide show presentation showing the initial models, initial dockings and final results of flexible fitting (PPT 2129 kb)
Supplementary Movie 1
Shows a tilt series of one of the myosin V 2-D arrays and an image of the tomogram obtained from it. (MOV 8635 kb)
Supplementary Movie 2
Shows the flower motif density envelope and the fitted atomic model with rotation and different degrees of zoom. (MOV 9217 kb)
Rights and permissions
About this article
Cite this article
Liu, J., Taylor, D., Krementsova, E. et al. Three-dimensional structure of the myosin V inhibited state by cryoelectron tomography. Nature 442, 208–211 (2006). https://doi.org/10.1038/nature04719
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature04719
This article is cited by
-
Myosin Va-dependent Transport of NMDA Receptors in Hippocampal Neurons
Neuroscience Bulletin (2024)
-
Autoregulation and dual stepping mode of MYA2, an Arabidopsis myosin XI responsible for cytoplasmic streaming
Scientific Reports (2022)
-
Arabidopsis thaliana myosin XIK is recruited to the Golgi through interaction with a MyoB receptor
Communications Biology (2021)
-
Regulation of class V myosin
Cellular and Molecular Life Sciences (2018)
-
Loss of Myosin Vb in colorectal cancer is a strong prognostic factor for disease recurrence
British Journal of Cancer (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.