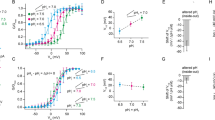
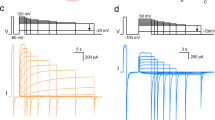
Abstract
Voltage changes across the cell membrane control the gating of many cation-selective ion channels. Conserved from bacteria to humans1, the voltage-gated-ligand superfamily of ion channels are encoded as polypeptide chains of six transmembrane-spanning segments (S1–S6). S1–S4 functions as a self-contained voltage-sensing domain (VSD), in essence a positively charged lever that moves in response to voltage changes. The VSD ‘ligand’ transmits force via a linker to the S5–S6 pore domain ‘receptor’2, thereby opening or closing the channel. The ascidian VSD protein Ci-VSP gates a phosphatase activity rather than a channel pore, indicating that VSDs function independently of ion channels3. Here we describe a mammalian VSD protein (HV1) that lacks a discernible pore domain but is sufficient for expression of a voltage-sensitive proton-selective ion channel activity. Hv1 currents are activated at depolarizing voltages, sensitive to the transmembrane pH gradient, H+-selective, and Zn2+-sensitive. Mutagenesis of Hv1 identified three arginine residues in S4 that regulate channel gating and two histidine residues that are required for extracellular inhibition of Hv1 by Zn2+. Hv1 is expressed in immune tissues and manifests the characteristic properties of native proton conductances (). In phagocytic leukocytes4, are required to support the oxidative burst that underlies microbial killing by the innate immune system4,5. The data presented here identify Hv1 as a long-sought voltage-gated H+ channel and establish Hv1 as the founding member of a family of mammalian VSD proteins.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Yu, F. H. & Catterall, W. A. The VGL-chanome: a protein superfamily specialized for electrical signaling and ionic homeostasis. Sci. STKE 2004, re15 (2004)
Long, S. B., Campbell, E. B. & Mackinnon, R. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science 309, 897–903 (2005)
Murata, Y., Iwasaki, H., Sasaki, M., Inaba, K. & Okamura, Y. Phosphoinositide phosphatase activity coupled to an intrinsic voltage sensor. Nature 435, 1239–1243 (2005)
DeCoursey, T. E. Voltage-gated proton channels and other proton transfer pathways. Physiol. Rev. 83, 475–579 (2003)
DeCoursey, T. E., Morgan, D. & Cherny, V. V. The voltage dependence of NADPH oxidase reveals why phagocytes need proton channels. Nature 422, 531–534 (2003)
Goldberg, J. et al. Three-dimensional structure of the catalytic subunit of protein serine/threonine phosphatase-1. Nature 376, 745–753 (1995)
Schilling, T., Gratopp, A., DeCoursey, T. E. & Eder, C. Voltage-activated proton currents in human lymphocytes. J. Physiol. (Lond.) 545, 93–105 (2002)
DeCoursey, T. E., Cherny, V. V., DeCoursey, A. G., Xu, W. & Thomas, L. L. Interactions between NADPH oxidase-related proton and electron currents in human eosinophils. J. Physiol. (Lond.) 535, 767–781 (2001)
Thomas, R. C. & Meech, R. W. Hydrogen ion currents and intracellular pH in depolarized voltage-clamped snail neurones. Nature 299, 826–828 (1982)
Henderson, L. M., Chappell, J. B. & Jones, O. T. Superoxide generation by the electrogenic NADPH oxidase of human neutrophils is limited by the movement of a compensating charge. Biochem. J. 255, 285–290 (1988)
Rada, B. K., Geiszt, M., Kaldi, K., Timar, C. & Ligeti, E. Dual role of phagocytic NADPH oxidase in bacterial killing. Blood 104, 2947–2953 (2004)
Babior, B. M. NADPH oxidase. Curr. Opin. Immunol. 16, 42–47 (2004)
Smith, R. M. & Curnutte, J. T. Molecular basis of chronic granulomatous disease. Blood 77, 673–686 (1991)
DeCoursey, T. E. & Cherny, V. V. Temperature dependence of voltage-gated H+ currents in human neutrophils, rat alveolar epithelial cells, and mammalian phagocytes. J. Gen. Physiol. 112, 503–522 (1998)
Cherny, V. V. & DeCoursey, T. E. pH-dependent inhibition of voltage-gated H+ currents in rat alveolar epithelial cells by Zn2+ and other divalent cations. J. Gen. Physiol. 114, 819–838 (1999)
Eder, C. & DeCoursey, T. E. Voltage-gated proton channels in microglia. Prog. Neurobiol. 64, 277–305 (2001)
Cherny, V. V., Henderson, L. M. & DeCoursey, T. E. Proton and chloride currents in Chinese hamster ovary cells. Membr. Cell Biol. 11, 337–347 (1997)
Morgan, D., Cherny, V. V., Price, M. O., Dinauer, M. C. & DeCoursey, T. E. Absence of proton channels in COS-7 cells expressing functional NADPH oxidase components. J. Gen. Physiol. 119, 571–580 (2002)
Bezanilla, F. The voltage sensor in voltage-dependent ion channels. Physiol. Rev. 80, 555–592 (2000)
Tombola, F., Pathak, M. M. & Isacoff, E. Y. How far will you go to sense voltage? Neuron 48, 719–725 (2005)
Starace, D. M. & Bezanilla, F. A proton pore in a potassium channel voltage sensor reveals a focused electric field. Nature 427, 548–553 (2004)
Pomes, R. & Roux, B. Molecular mechanism of H+ conduction in the single-file water chain of the gramicidin channel. Biophys. J. 82, 2304–2316 (2002)
DeCoursey, T. E., Morgan, D. & Cherny, V. V. The gp91phox component of NADPH oxidase is not a voltage-gated proton channel. J. Gen. Physiol. 120, 773–779 (2002)
Henderson, L. M. & Meech, R. W. Evidence that the product of the human X-linked CGD gene, gp91-phox, is a voltage-gated H+ pathway. J. Gen. Physiol. 114, 771–786 (1999)
Bokoch, G. M. Regulation of innate immunity by Rho GTPases. Trends Cell Biol. 15, 163–171 (2005)
DeCoursey, T. E., Cherny, V. V., Morgan, D., Katz, B. Z. & Dinauer, M. C. The gp91phox component of NADPH oxidase is not the voltage-gated proton channel in phagocytes, but it helps. J. Biol. Chem. 276, 36063–36066 (2001)
Price, M. O. et al. Creation of a genetic system for analysis of the phagocyte respiratory burst: high-level reconstitution of the NADPH oxidase in a nonhematopoietic system. Blood 99, 2653–2661 (2002)
Bokoch, G. M. & Knaus, U. G. NADPH oxidases: not just for leukocytes anymore! Trends Biochem. Sci. 28, 502–508 (2003)
Qu, A. Y., Nanda, A., Curnutte, J. T. & Grinstein, S. Development of a H+-selective conductance during granulocytic differentiation of HL-60 cells. Am. J. Physiol. 266, C1263–C1270 (1994)
Sasaki, M., Takagi, M. & Okamura, Y. A novel protein with a voltage sensor domain is a voltage-gated proton channel. Science (in the press)
Acknowledgements
We thank T. DeCoursey, C. Miller, R. MacKinnon and P. Bezanilla for comments on the manuscript, and K.-H. Lee for technical assistance. This work was supported by the Sandler Program for Asthma Research and the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
Supplementary information
Supplementary Figure Legends
This file contains text to accompany the Supplementary Figures. (DOC 56 kb)
Supplementary Figure 1
Hv1 amino acid sequence and expression profile. (PDF 1523 kb)
Supplementary Figure 2
a, Human tissue Western blot probed with 4234 antibody (5 µg/ml) demonstrates expression of native Hv1 protein (~32 kDa) in immune tissues. b, Western blot of total cell lysates prepared from Jurkat (lane 1) or HEK-293T cells transfected with the indicated cDNA (lanes 3-5). c, in HL-60 cells, Hv1 protein appeared to be increased when cells were cultured in the presence of 1.3% DMSO (lane 2). (PDF 1536 kb)
Supplementary Figure 3
a, Hv1-like currents were not detectable in HM1 cells. b, Native GvH+ in a DMSO-differentiated HL-60 cell. c, Temperature dependence of Hv1 currents. d, Monoexponential fits of τACT from the same cell as shown in panel c illustrate strong temperature-dependence of Hv1 kinetics. e, R205A currents. f, R208A currents. g, R211A currents. (PDF 2674 kb)
Rights and permissions
About this article
Cite this article
Ramsey, I., Moran, M., Chong, J. et al. A voltage-gated proton-selective channel lacking the pore domain. Nature 440, 1213–1216 (2006). https://doi.org/10.1038/nature04700
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature04700
This article is cited by
-
Control of intracellular pH and bicarbonate by CO2 diffusion into human sperm
Nature Communications (2023)
-
Vertebrate OTOP1 is also an alkali-activated channel
Nature Communications (2023)
-
Ion currents through the voltage sensor domain of distinct families of proteins
Journal of Biological Physics (2023)
-
Role of the Voltage-Gated Proton Channel Hv1 in Nervous Systems
Neuroscience Bulletin (2023)
-
Multiple mechanisms contribute to fluorometry signals from the voltage-gated proton channel
Communications Biology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.