Abstract
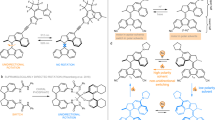
Molecular analogues of a variety of mechanical devices such as shuttles, brakes, unidirectional rotors and tweezers have been created1,2,3,4,5,6,7,8,9,10,11. But these ‘molecular machines’ have not yet been used to mechanically manipulate a second molecule in a controlled and reversible manner. Here we show that light-induced scissor-like conformational changes of one molecule5 can give rise to mechanical twisting of a non-covalently bound guest molecule. To realize this coupling of molecular motions, we use a previously designed system5: a ferrocene moiety with an azobenzene strap, each end of which is attached to one of the two cyclopentadienyl rings of the ferrocene unit, acts as a pivot so that photoisomerization of the strap rotates the ferrocene rings relative to each other and thereby also changes the relative position of two ‘pedal’ moieties attached to the ferrocene rings. We translate this effect into intermolecular coupling of motion by endowing the pedals with binding sites, which allow the host system to form a stable complex with a bidentate rotor molecule. Using circular dichroism spectroscopy, we show that the photoinduced conformational changes of the host are indeed transmitted and induce mechanical twisting of the rotor molecule. This design concept, which significantly extends the successful coupling of motion beyond the intramolecular level seen in synthetic allosteric receptors12, might allow for the remote control of molecular events in larger interlocked molecular systems.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kinbara, K. & Aida, T. Toward intelligent molecular machines: directed motions of biological and artificial molecules and assemblies. Chem. Rev. 105, 1377–1400 (2005)
Collin, J.-P., Dietrich-Buchecker, C., Gaviña, P., Jimenez-Molero, M. C. & Sauvage, J.-P. Shuttles and muscles: linear molecular machines based on transition metals. Acc. Chem. Res. 34, 477–487 (2001)
Balzani, V., Credi, A., Raymo, F. M. & Stoddart, J. F. Artificial molecular machines. Angew. Chem. Int. Edn Engl. 39, 3348–3391 (2000)
Badjic, J. D., Balzani, V., Credi, A., Silvi, S. & Stoddart, J. F. A molecular elevator. Science 303, 1845–1849 (2004)
Muraoka, T., Kinbara, K., Kobayashi, Y. & Aida, T. Light-driven open–close motion of chiral molecular scissors. J. Am. Chem. Soc. 125, 5612–5613 (2003)
Ishii, D. et al. Chaperonin-mediated stabilization and ATP-triggered release of semiconductor nanoparticles. Nature 423, 628–632 (2003)
Leigh, D. A., Wong, J. K. Y., Dehez, F. & Zerbetto, F. Unidirectional rotation in a mechanically interlocked molecular rotor. Nature 424, 174–179 (2003)
Tashiro, K., Konishi, K. & Aida, T. Metal bisporphyrinate double-decker complexes as redox-responsive rotating modules. Studies on ligand rotation activities of the reduced and oxidized forms using chirality as a probe. J. Am. Chem. Soc. 122, 7921–7926 (2000)
Kelly, T. R., De Silva, H. & Silva, R. A. Unidirectional rotary motion in a molecular system. Nature 401, 150–152 (1999)
Koumura, N., Zijlstra, R. W. J., van Delden, R. A., Harada, N. & Feringa, B. L. Light-driven monodirectional molecular rotor. Nature 401, 152–155 (1999)
Shinkai, S., Ogawa, T., Nakaji, T., Kusano, Y. & Manabe, O. Photocontrolled extraction ability of azobenzene-bridged azacrown ether. Tetrahedr. Lett. 20, 4569–4572 (1979)
Rebek, J. & Wattley, R. V. Allosteric effects: remote control of ion transport selectivity. J. Am. Chem. Soc. 102, 4853–4854 (1980)
Guo, Y.-M., Oike, H. & Aida, T. Chiroptical transcription of helical information through supramolecular harmonization with dynamic helices. J. Am. Chem. Soc. 126, 716–717 (2004)
Borovkov, V. V., Hembury, G. A. & Inoue, Y. Origin, control, and application of supramolecular chirogenesis in bisporphyrin-based systems. Acc. Chem. Res. 37, 449–459 (2004)
Huang, X. et al. Absolute configurational assignments of secondary amines by CD-sensitive dimeric zinc porphyrin host. J. Am. Chem. Soc. 124, 10320–10335 (2002)
Harada, N., Chen, S. L. & Nakanishi, K. Quantitative definition of exciton chirality and the distant effect in the exciton chirality method. J. Am. Chem. Soc. 97, 5345–5352 (1975)
Hanazaki, I. & Akimoto, H. Optical rotatory power of 2,2′-dihydroxy-1,1′-binaphthyl and related compounds. J. Am. Chem. Soc. 94, 4102–4106 (1972)
Tamai, N. & Miyasaka, H. Ultrafast dynamics of photochromic system. Chem. Rev. 100, 1875–1890 (2000)
Spörlein, S. et al. Ultrafast spectroscopy reveals subnanosecond peptide conformational dynamics and validates molecular dynamics simulation. Proc. Natl Acad. Sci. USA 99, 7998–8002 (2002)
Wachtveitl, J. et al. Ultrafast conformational dynamics in cyclic azobenzene peptides of increased flexibility. Biophys. J. 86, 2350–2362 (2004)
Gardner, A. B., Howard, J., Waddington, T. C., Richardson, R. M. & Tomkinson, J. The dynamics of ring rotation in ferrocene, nickelocene, and ruthenocene by incoherent quasi-elastic neutron scattering. Chem. Phys. 57, 453–460 (1981)
Stephenson, D. S. & Binsch, G. Improved algorism for the computation of exchange-broadened NMR bandshapes. J. Magn. Reson. 30, 625–626 (1978)
Acknowledgements
T.M. thanks the JSPS Young Scientist Fellowship. This research was partially supported by the Ministry of Education, Science, Sports and Culture, Grant-in-Aid for Scientific Research on Priority Areas ‘Life-Surveyors’ (to K.K.). We thank Y. Casta and S. Nara for cooperation in synthesis, and N. Tamai and M. Irie for discussion on photophysics.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
Supplementary information
Supplementary Notes
This file contains the Supplementary Methods (experimental details for synthesis and measurements) and Supplementary Figures 1-12. (PDF 1867 kb)
Supplementary Movie 1
This movie represents computer-generated 3D molecular models of 2otrans-1 and 2ocis-1 viewed from various angles. (MOV 9094 kb)
Rights and permissions
About this article
Cite this article
Muraoka, T., Kinbara, K. & Aida, T. Mechanical twisting of a guest by a photoresponsive host. Nature 440, 512–515 (2006). https://doi.org/10.1038/nature04635
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature04635
This article is cited by
-
Selective synthesis of tightly- and loosely-twisted metallomacrocycle isomers towards precise control of helicity inversion motion
Nature Communications (2023)
-
Synthesis of planar chiral ferrocenes via enantioselective remote C–H activation
Nature Chemistry (2023)
-
Moving forward in the semantic soup of artificial molecular machine taxonomy
Nature Nanotechnology (2022)
-
Photogearing as a concept for translation of precise motions at the nanoscale
Nature Chemistry (2022)
-
Photoinduced plasticizing effect of the addition of azobenzene on the glass transition temperature and mechanical properties of polycarbonate
Polymer Journal (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.