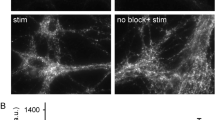
Abstract
Synaptic transmission is mediated by neurotransmitters that are stored in synaptic vesicles and released by exocytosis upon activation. The vesicle membrane is then retrieved by endocytosis, and synaptic vesicles are regenerated and re-filled with neurotransmitter1. Although many aspects of vesicle recycling are understood, the fate of the vesicles after fusion is still unclear. Do their components diffuse on the plasma membrane, or do they remain together? This question has been difficult to answer because synaptic vesicles are too small (∼40 nm in diameter) and too densely packed to be resolved by available fluorescence microscopes. Here we use stimulated emission depletion (STED)2 to reduce the focal spot area by about an order of magnitude below the diffraction limit, thereby resolving individual vesicles in the synapse. We show that synaptotagmin I, a protein resident in the vesicle membrane, remains clustered in isolated patches on the presynaptic membrane regardless of whether the nerve terminals are mildly active or intensely stimulated. This suggests that at least some vesicle constituents remain together during recycling. Our study also demonstrates that questions involving cellular structures with dimensions of a few tens of nanometres can be resolved with conventional far-field optics and visible light.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Sudhof, T. C. The synaptic vesicle cycle. Annu. Rev. Neurosci. 27, 509–547 (2004)
Hell, S. W. & Wichmann, J. Breaking the diffraction resolution limit by stimulated emission: stimulated emission depletion microscopy. Opt. Lett. 19, 780–782 (1994)
Ceccarelli, B., Hurlbut, W. P. & Mauro, A. Turnover of transmitter and synaptic vesicles at frog neuromuscular junction. J. Cell Biol. 57, 499–524 (1973)
Heuser, J. E. & Reese, T. S. Evidence for recycling of synaptic vesicle membrane during transmitter release at frog neuromuscular junction. J. Cell Biol. 57, 315–344 (1973)
Rizzoli, S. O. & Betz, W. J. Synaptic vesicle pools. Nature Rev. Neurosci. 6, 57–69 (2005)
Royle, S. J. & Lagnado, L. Endocytosis at the synaptic terminal. J. Physiol. (Lond.) 553, 345–355 (2003)
Gandhi, S. P. & Stevens, C. F. Three modes of synaptic vesicular recycling revealed by single-vesicle imaging. Nature 423, 607–613 (2003)
Aravanis, A. M., Pyle, J. L. & Tsien, R. W. Single synaptic vesicles fusing transiently and successively without loss of identity. Nature 423, 643–647 (2003)
Zenisek, D., Steyer, J. A., Feldman, M. E. & Almers, W. A membrane marker leaves synaptic vesicles in milliseconds after exocytosis in retinal bipolar cells. Neuron 35, 1085–1097 (2002)
Klar, T. A., Jakobs, S., Dyba, M., Egner, A. & Hell, S. W. Fluorescence microscopy with diffraction resolution limit broken by stimulated emission. Proc. Natl Acad. Sci. USA 97, 8206–8210 (2000)
Hell, S. W. Toward fluorescence nanoscopy. Nature Biotechnol. 21, 1347–1355 (2003)
Hell, S. W. in Topics in Fluorescence Spectroscopy (ed. Lakowicz, J. R.) 361–422 (Plenum Press, New York, 1997)
Hell, S. W. Strategy for far-field optical imaging and writing without diffraction limit. Phys. Lett. A 326, 140–145 (2004)
Wilson, T. & Sheppard, C. J. R. Theory and Practice of Scanning Optical Microscopy (Academic, New York, 1984)
Chapman, E. R. & Jahn, R. Calcium-dependent interaction of the cytoplasmic region of synaptotagmin with membranes. Autonomous function of a single C2-homologous domain. J. Biol. Chem. 269, 5735–5741 (1994)
Kraszewski, K. et al. Synaptic vesicle dynamics in living cultured hippocampal-neurons visualized with Cy3-conjugated antibodies directed against the lumenal domain of synaptotagmin. J. Neurosci. 15, 4328–4342 (1995)
Brose, N., Petrenko, A. G., Sudhof, T. C. & Jahn, R. Synaptotagmin: a calcium sensor on the synaptic vesicle surface. Science 256, 1021–1025 (1992)
Pyle, J. L., Kavalali, E. T., Piedras-Renteria, E. S. & Tsien, R. W. Rapid reuse of readily releasable pool vesicles at hippocampal synapses. Neuron 28, 221–231 (2000)
Harris, K. M. & Sultan, P. Variation in the number, location and size of synaptic vesicles provides an anatomical basis for the nonuniform probability of release at hippocampal CA1 synapses. Neuropharmacology 34, 1387–1395 (1995)
Sankaranarayanan, S. & Ryan, T. A. Real-time measurements of vesicle-SNARE recycling in synapses of the central nervous system. Nature Cell Biol. 2, 197–204 (2000)
Poskanzer, K. E., Marek, K. W., Sweeney, S. T. & Davis, G. W. Synaptotagmin I is necessary for compensatory synaptic vesicle endocytosis in vivo. Nature 426, 559–563 (2003)
Bennett, M., Calakos, N., Kreiner, T. & Scheller, R. Synaptic vesicle membrane proteins interact to form a multimeric complex. J. Cell Biol. 116, 761–775 (1992)
Westphal, V. & Hell, S. W. Nanoscale resolution in the focal plane of an optical microscope. Phys. Rev. Lett. 94, 143903 (2005)
Rosenmund, C. & Stevens, C. F. The rate of aldehyde fixation of the exocytotic machinery in cultured hippocampal synapses. J. Neurosci. Methods 76, 1–5 (1997)
Klingauf, J., Kavalali, E. T. & Tsien, R. W. Kinetics and regulation of fast endocytosis at hippocampal synapses. Nature 394, 581–585 (1998)
Török, P. & Munro, P. R. T. The use of Gauss-Laguerre vector beams in STED microscopy. Opt. Expr. 12, 3605–3617 (2004)
Acknowledgements
The authors thank E. Neher for helpful comments. S.O.R. acknowledges fellowships from the European Molecular Biology Organization and from the Human Frontier Science Program. This work was partly supported by a grant from the Leibniz Program of the Deutsche Forschungsgemeinschaft awarded to R.J., a grant from the German Ministry of Research and Education to S.W.H., and by the DFG-Centre for Molecular Physiology of the Brain. We thank I. Herefort, M. Wienisch and J. Klingauf for assistance with cell culturing, and A. Schönle for help with his software ImSpector.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
Supplementary information
Supplementary Figure S1
Bright (putatively surface) fluorescent dots persist in permeabilized preparations. (JPG 27 kb)
Supplementary Figure S2
Quantification of dot half-width using different dot-selection approaches. (JPG 47 kb)
Supplementary Figure S3
Alternative models for the recycling of synaptotagmin. (JPG 21 kb)
Supplementary Figure S4
The pool of surface synaptotagmin can undergo vesicle recycling. (JPG 21 kb)
Supplementary Figure Legends
This file contains full legends for Supplementary Figures S1–S4. (DOC 22 kb)
Rights and permissions
About this article
Cite this article
Willig, K., Rizzoli, S., Westphal, V. et al. STED microscopy reveals that synaptotagmin remains clustered after synaptic vesicle exocytosis. Nature 440, 935–939 (2006). https://doi.org/10.1038/nature04592
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature04592
This article is cited by
-
Structural basis for antiepileptic drugs and botulinum neurotoxin recognition of SV2A
Nature Communications (2024)
-
Membrane transformations of fusion and budding
Nature Communications (2024)
-
Far-field super-resolution chemical microscopy
Light: Science & Applications (2023)
-
Broadband optical vortex beam generation using flat-surface nanostructured gradient index vortex phase masks
Scientific Reports (2023)
-
Tunable photon-induced spatial modulation of free electrons
Nature Materials (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.