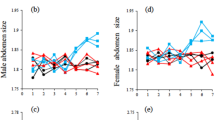
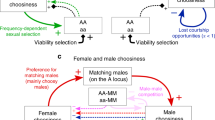
Abstract
One of the most debated questions in evolutionary biology is whether female choice of males with exaggerated sexual displays can evolve as a correlated response to selection acting on genes coding for male attractiveness or high overall viability. To date, empirical studies have provided support for parts of this scenario1, but evidence for all key genetic components in a natural population is lacking. Here we use animal-model quantitative genetic analysis2,3 on data from over 8,500 collared flycatchers (Ficedula albicollis) followed for 24 years to quantify all of the key genetic requirements of both fisherian4,5 and ‘good-genes’ models6,7 on sexual selection in the wild. We found significant additive genetic variances of all the main components: male ornament (forehead patch size), female mate choice for this ornament, male fitness and female fitness. However, when the necessary genetic correlations between these components were taken into account, the estimated strength of indirect sexual selection on female mate choice was negligible. Our results show that the combined effect of environmental influences on several components reduces the potential for indirect sexual selection in the wild. This study provides insight into the field of sexual selection by showing that genes coding for mate choice for an ornament probably evolve by their own pathways instead of ‘hitchhiking’ with genes coding for the ornament.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Kokko, H., Brooks, R., Jennions, M. D. & Morley, J. The evolution of mate choice and mating biases. Proc. R. Soc. Lond. B 270, 653–664 (2003)
Lynch, M. & Walsh, B. Genetics and Analysis of Quantitative Traits (Sinauer, Sutherland, Massachusetts, 1996)
Kruuk, L. E. B. Estimating genetic parameters in natural populations using the ‘animal model’. Phil. Trans. R. Soc. Lond. B 359, 873–890 (2004)
Fisher, R. A. The Genetical Theory of Natural Selection (Clarendon Press, London, 1930)
Lande, R. Models of speciation by sexual selection on polygenic traits. Proc. Natl Acad. Sci. USA 78, 3721–3725 (1981)
Zahavi, A. Mate selection: a selection for a handicap. J. Theor. Biol. 53, 205–214 (1975)
Iwasa, Y., Pomiankowski, A. & Nee, S. The evolution of costly mate preferences. II. The handicap principle. Evolution 45, 1431–1442 (1991)
Andersson, M. Sexual Selection (Princeton Univ. Press, Princeton, 1994)
Bakker, T. C. M. Positive genetic correlation between female preference and preferred male ornament in sticklebacks. Nature 363, 255–257 (1993)
Iyengar, V. K., Reeve, H. K. & Eisner, T. Paternal inheritance of a female moth's mating preference: consequences for sexual selection. Nature 419, 830–832 (2002)
Houde, A. E. Effect on artificial selection on male colour patterns on mating preference of female guppies. Proc. R. Soc. Lond. B 256, 125–130 (1994)
Brooks, R. & Couldridge, V. Multiple sexual ornaments coevolve with multiple mating preferences. Am. Nat. 154, 37–45 (1999)
Hine, E., Lachish, S., Higgie, M. & Blows, M. W. Positive genetic correlation between female preference and offspring fitness. Proc. R. Soc. Lond. B 269, 2215–2219 (2002)
Kirkpatrick, M. & Barton, N. H. The strength of indirect selection on female mating preferences. Proc. Natl Acad. Sci. USA 94, 1282–1286 (1997)
Service, P. M. & Rose, M. R. Genetic covariation among life history components: the effect of novel environments. Evolution 39, 943–945 (1985)
Pärt, T. & Qvarnström, A. Badge size in collared flycatchers predicts outcome of male competition over territories. Anim. Behav. 54, 893–899 (1997)
Qvarnström, A. Experimentally increased badge size increases male competition and reduces male parental care in the collared flycatcher. Proc. R. Soc. Lond. B 264, 1225–1231 (1997)
Sheldon, B. C., Merilä, J., Qvarnström, A., Gustafsson, L. & Ellegren, H. Paternal genetic contribution to offspring condition predicted by size of male secondary sexual character. Proc. R. Soc. Lond. B 264, 297–302 (1997)
Qvarnström, A., Pärt, T. & Sheldon, B. C. Adaptive plasticity in mate preference linked to differences in reproductive effort. Nature 405, 344–347 (2000)
Qvarnström, A. Genotype-by-environment interactions in the determination of the size of a secondary sexual character in the collared flycatcher (Ficedula albicollis). Evolution 53, 1564–1572 (1999)
Garant, D., Sheldon, B. C. & Gustafsson, L. Climatic and temporal effects on the expression of secondary sexual characters: genetic and environmental components. Evolution 58, 634–644 (2004)
Pomiankowski, A. & Møller, A. P. A resolution of the lek paradox. Proc. R. Soc. Lond. B 260, 21–29 (1995)
Burt, A. Perspective: the evolution of fitness. Evolution 49, 1–8 (1995)
Höglund, J. & Alatalo, R. V. Leks (Princeton Univ. Press, Princeton, 1995)
Bakker, T. C. M. The study of intersexual selection using quantitative genetics. Behaviour 136, 1237–1266 (1999)
Kruuk, L. E. B. et al. Heritability of fitness in a wild mammal population. Proc. Natl Acad. Sci. USA 97, 698–703 (2000)
Dale, S., Rinden, H. & Slagsvold, T. Competition for a mate restricts mate search of female flycatchers. Behav. Ecol. Sociobiol. 30, 165–176 (1992)
Lesna, I. & Sabelis, M. W. Diet-dependent female choice for males with ‘good genes’ in a soil predatory mite. Nature 401, 581–584 (1999)
Hunt, J., Brooks, R. & Jennions, M. D. Female mate choice as a condition-dependent life-history trait. Am. Nat. 166, 79–92 (2005)
Charmantier, A. & Reale, D. How do misassigned paternities affect the estimation of heritabilities in the wild? Mol. Ecol. 14, 2839–2850 (2005)
Acknowledgements
We thank A. Berglund, M. Björklund, M. Kirkpatrick and T. Pärt for comments on the manuscript and for technical advice, and all people involved in field work over the years. Financial support was provided by the Swedish Research Council (to A.Q. and L.G.) and the Academy of Finland (to J.E.B.). Author Contributions A.Q. came up with the idea and wrote the manuscript together with J.E.B, who also did all the analyses. L.G. organized the long-term study, and all three authors took active part in discussion and commenting on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
Supplementary information
Supplementary Notes
This file contains the Supplementary Methods and additional references. (PDF 121 kb)
Rights and permissions
About this article
Cite this article
Qvarnström, A., Brommer, J. & Gustafsson, L. Testing the genetics underlying the co-evolution of mate choice and ornament in the wild. Nature 441, 84–86 (2006). https://doi.org/10.1038/nature04564
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature04564
This article is cited by
-
Measuring fitness and inferring natural selection from long-term field studies: different measures lead to nuanced conclusions
Behavioral Ecology and Sociobiology (2022)
-
Climate change upends selection on ornamentation in a wild bird
Nature Ecology & Evolution (2017)
-
The Role of Genes and Environment in Degree of Partner Self-Similarity
Behavior Genetics (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.