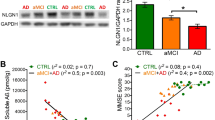
Abstract
Memory function often declines with age1, and is believed to deteriorate initially because of changes in synaptic function rather than loss of neurons2. Some individuals then go on to develop Alzheimer's disease with neurodegeneration. Here we use Tg2576 mice, which express a human amyloid-β precursor protein (APP) variant linked to Alzheimer's disease, to investigate the cause of memory decline in the absence of neurodegeneration or amyloid-β protein amyloidosis. Young Tg2576 mice (< 6 months old) have normal memory and lack neuropathology, middle-aged mice (6–14 months old) develop memory deficits without neuronal loss, and old mice (> 14 months old) form abundant neuritic plaques containing amyloid-β (refs 3–6). We found that memory deficits in middle-aged Tg2576 mice are caused by the extracellular accumulation of a 56-kDa soluble amyloid-β assembly, which we term Aβ*56 (Aβ star 56). Aβ*56 purified from the brains of impaired Tg2576 mice disrupts memory when administered to young rats. We propose that Aβ*56 impairs memory independently of plaques or neuronal loss, and may contribute to cognitive deficits associated with Alzheimer's disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
14 July 2022
Editor’s Note: The editors of Nature have been alerted to concerns regarding some of the figures in this paper. Nature is investigating these concerns, and a further editorial response will follow as soon as possible. In the meantime, readers are advised to use caution when using results reported therein.
References
Craik, F. I. in Handbook of the Psychology of Aging (eds Birren, J. E. & Schall, K.) 384–420 (Van Nostrand-Reinhold, New York, 1977)
Morrison, J. H. & Hof, P. R. Life and death of neurons in the aging brain. Science 278, 412–419 (1997)
Hsiao, K. et al. Correlative memory deficits, Aβ elevation, and amyloid plaques in transgenic mice. Science 274, 99–102 (1996)
Irizarry, M. C., McNamara, M., Fedorchak, K., Hsiao, K. & Hyman, B. T. APPSW transgenic mice develop age-related Aβ deposits and neuropil abnormalities, but no neuronal loss in CA1. J. Neuropathol. Exp. Neurol. 56, 965–973 (1997)
Kawarabayashi, T. et al. Age-dependent changes in brain, CSF, and plasma amyloid β protein in the Tg2576 transgenic mouse model of Alzheimer's disease. J. Neurosci. 21, 372–381 (2001)
Westerman, M. A. et al. The relationship between Aβ and memory in the Tg2576 mouse model of Alzheimer's disease. J. Neurosci. 22, 1858–1867 (2002)
Kawas, C. H. et al. Visual memory predicts Alzheimer's disease more than a decade before diagnosis. Neurology 60, 1089–1093 (2003)
Small, G. W. et al. Cerebral metabolic and cognitive decline in persons at genetic risk for Alzheimer's disease. Proc. Natl Acad. Sci. USA 97, 6037–6042 (2000)
Bookheimer, S. Y. et al. Patterns of brain activation in people at risk for Alzheimer's disease. N. Engl. J. Med. 343, 450–456 (2000)
Albert, M. S. Memory decline: the boundary between aging and age-related disease. Ann. Neurol. 51, 282–284 (2002)
Benzing, W. C. et al. Evidence for glial-mediated inflammation in aged APPSW transgenic mice. Neurobiol. Aging 20, 581–589 (1999)
Janus, C. et al. Aβ peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer's disease. Nature 408, 979–982 (2000)
Chen, G. et al. A learning deficit related to age and β-amyloid plaques in a mouse model of Alzheimer's disease. Nature 408, 975–979 (2000)
Hsia, A. Y. et al. Plaque-independent disruption of neural circuits in Alzheimer's disease mouse models. Proc. Natl Acad. Sci. USA 96, 3228–3233 (1999)
Klein, W. L., Krafft, G. A. & Finch, C. E. Targeting small Aβ oligomers: the solution to an Alzheimer's disease conundrum? Trends Neurosci. 24, 219–224 (2001)
Ashe, K. H. Learning and memory in transgenic mice modeling Alzheimer's disease. Learn. Mem. 8, 301–308 (2001)
Gordon, J. A. & Warren, J. R. Denaturation of globular proteins. I. The interaction of urea and thiourea with bovine plasma albumin. J. Biol. Chem. 243, 5663–5669 (1968)
Kayed, R. et al. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science 300, 486–489 (2003)
Stern, E. A. et al. Cortical synaptic integration in vivo is disrupted by amyloid-β plaques. J. Neurosci. 24, 4535–4540 (2004)
Billings, L. M., Oddo, S., Green, K. N., McGaugh, J. L. & Laferla, F. M. Intraneuronal Aβ causes the onset of early Alzheimer's disease-related cognitive deficits in transgenic mice. Neuron 45, 675–688 (2005)
Orr, H. T. Neurodegenerative disease: neuron protection agency. Nature 431, 747–748 (2004)
SantaCruz, K. et al. Tau suppression in a neurodegenerative mouse model improves memory function. Science 309, 476–481 (2005)
Duff, K. & Planel, E. Untangling memory deficits. Nature Med. 11, 826–827 (2005)
Gallagher, M., Burwell, R. & Burchinal, M. Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behav. Neurosci. 107, 618–626 (1993)
Walsh, D. M. et al. Naturally secreted oligomers of amyloid β protein potently inhibit hippocampal long-term potentiation in vivo. Nature 416, 535–539 (2002)
Haass, C., Koo, E. H., Mellon, A., Hung, A. Y. & Selkoe, D. J. Targeting of cell-surface β-amyloid precursor protein to lysosomes: alternative processing into amyloid-bearing fragments. Nature 357, 500–503 (1992)
Barelli, H. et al. Characterization of new polyclonal antibodies specific for 40 and 42 amino acid-long amyloid β peptides: their use to examine the cell biology of presenilins and the immunohistochemistry of sporadic Alzheimer's disease and cerebral amyloid angiopathy cases. Mol. Med. 3, 695–707 (1997)
Sergeant, N. et al. Progressive decrease of amyloid precursor protein carboxy terminal fragments (APP-CTFs), associated with tau pathology stages, in Alzheimer's disease. J. Neurochem. 81, 663–672 (2002)
Acknowledgements
We thank S. Younkin, D. Walsh, M. Podlisny, D. Selkoe, J. Cleary and S. Prusiner for critical discussions. We are grateful to D. Cooper-Blacketer, J. McQuail and M. Sherman for technical help, and A. Delacourte and N. Sergeant for providing the APPC17-Cter antiserum. This work was supported by grants from the NIH (to K.H.A., M.G. and A.Y.) and a gift from M. and H. Hobbs to M.G. Author Contributions S.L. and K.H.A. conceived the project. S.L. planned, performed and analysed the biochemistry experiments, including the protein extractions and purification of Aβ*56. K.H.A. wrote most of the paper. M.T.K. and M.G. planned, performed and analysed the rat behavioural experiments, and L.K. planned and analysed the mouse behavioural experiments. A.Y. provided the mass spectrometry analysis of purified Aβ*56, C.G.G. provided biochemistry advice, and C.G.G. and R.K. donated the A11 antiserum.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
S.L. and K.H.A. are inventors on a patent application submitted by the University of Minnesota that describes Aβ*56.
Supplementary information
Supplementary Notes
This file contains the Supplementary Methods, Supplementary Discussion (on the selective effects of Aβ*56 on retention, but not acquisition of spatial memory) Supplementary Table 1, Supplementary Figures 1–7 and additional references. (PDF 2010 kb)
Rights and permissions
About this article
Cite this article
Lesné, S., Koh, M., Kotilinek, L. et al. A specific amyloid-β protein assembly in the brain impairs memory. Nature 440, 352–357 (2006). https://doi.org/10.1038/nature04533
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature04533
This article is cited by
-
Investigation of encapsulated water wire within self-assembled hydrophilic nanochannels, in a modified γ4-amino acid crystals: Tracking thermally induced changes of intermolecular interactions within a crystalline hydrate
Amino Acids (2024)
-
Alleviating the unwanted effects of oxidative stress on Aβ clearance: a review of related concepts and strategies for the development of computational modelling
Translational Neurodegeneration (2023)
-
Activation of the IL-17/TRAF6/NF-κB pathway is implicated in Aβ-induced neurotoxicity
BMC Neuroscience (2023)
-
Lessons from Alzheimer's research
British Dental Journal (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.