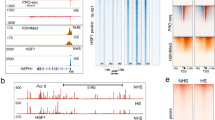
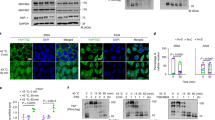
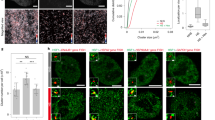
Abstract
The heat-shock transcription factor 1 (HSF1) has an important role in the heat-shock response in vertebrates by inducing the expression of heat-shock proteins (HSPs) and other cytoprotective proteins1. HSF1 is present in unstressed cells in an inactive monomeric form and becomes activated by heat and other stress stimuli. HSF1 activation involves trimerization and acquisition of a site-specific DNA-binding activity2,3, which is negatively regulated by interaction with certain HSPs4,5,6. Here we show that HSF1 activation by heat shock is an active process that is mediated by a ribonucleoprotein complex containing translation elongation factor eEF1A and a previously unknown non-coding RNA that we term HSR1 (heat shock RNA-1). HSR1 is constitutively expressed in human and rodent cells and its homologues are functionally interchangeable. Both HSR1 and eEF1A are required for HSF1 activation in vitro; antisense oligonucleotides or short interfering (si)RNA against HSR1 impair the heat-shock response in vivo, rendering cells thermosensitive. The central role of HSR1 during heat shock implies that targeting this RNA could serve as a new therapeutic model for cancer, inflammation and other conditions associated with HSF1 deregulation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Sarge, K. D., Zimarino, V., Holm, K., Wu, C. & Morimoto, R. I. Cloning and characterization of two mouse heat shock factors with distinct inducible and constitutive DNA-binding ability. Genes Dev. 5, 1902–1911 (1991)
Sarge, K. D., Murphy, S. P. & Morimoto, R. I. Activation of heat shock gene transcription by heat shock factor 1 involves oligomerization, acquisition of DNA-binding activity, and nuclear localization and can occur in the absence of stress. Mol. Cell. Biol. 13, 1392–1407 (1993)
Westwood, J. T. & Wu, C. Activation of Drosophila heat shock factor: conformational change associated with a monomer-to-trimer transition. Mol. Cell. Biol. 13, 3481–3486 (1993)
Zou, J., Guo, Y., Guettouche, T., Smith, D. F. & Voellmy, R. Repression of heat shock transcription factor HSF1 activation by HSP90 (HSP90 complex) that forms a stress-sensitive complex with HSF1. Cell 94, 471–480 (1998)
Voellmy, R. Transcriptional regulation of the metazoan stress protein response. Prog. Nucleic Acid Res. Mol. Biol. 78, 143–185 (2004)
Shi, Y., Mosser, D. D. & Morimoto, R. I. Molecular chaperones as HSF1-specific transcriptional repressors. Genes Dev. 12, 654–666 (1998)
Abravaya, K., Myers, M. P., Murphy, S. P. & Morimoto, R. I. The human heat shock protein hsp70 interacts with HSF, the transcription factor that regulates heat shock gene expression. Genes Dev. 6, 1153–1164 (1992)
Marchler, G. & Wu, C. Modulation of Drosophila heat shock transcription factor activity by the molecular chaperone DROJ1. EMBO J. 20, 499–509 (2001)
Zimarino, V., Tsai, C. & Wu, C. Complex modes of heat shock factor activation. Mol. Cell. Biol. 10, 752–759 (1990)
Shopland, L. S., Hirayoshi, K., Fernandes, M. & Lis, J. T. HSF access to heat shock elements in vivo depends critically on promoter architecture defined by GAGA factor, TFIID, and RNA polymerase II binding sites. Genes Dev. 9, 2756–2769 (1995)
Shopland, L. S. & Lis, J. T. HSF recruitment and loss at most Drosophila heat shock loci is coordinated and depends on proximal promoter sequences. Chromosoma 105, 158–171 (1996)
Zuo, J., Rungger, D. & Voellmy, R. Multiple layers of regulation of human heat shock transcription factor 1. Mol. Cell. Biol. 15, 4319–4330 (1995)
Ali, A., Bharadwaj, S., O'Carroll, R. & Ovsenek, N. HSP90 interacts with and regulates the activity of heat shock factor 1 in Xenopus oocytes. Mol. Cell. Biol. 18, 4949–4960 (1998)
Bharadwaj, S., Ali, A. & Ovsenek, N. Multiple components of the HSP90 chaperone complex function in regulation of heat shock factor 1 In vivo. Mol. Cell. Biol. 19, 8033–8041 (1999)
Clos, J., Rabindran, S., Wisniewski, J. & Wu, C. Induction temperature of human heat shock factor is reprogrammed in a Drosophila cell environment. Nature 364, 252–255 (1993)
Batulan, Z. et al. High threshold for induction of the stress response in motor neurons is associated with failure to activate HSF1. J. Neurosci. 23, 5789–5798 (2003)
Gothard, L. Q., Ruffner, M. E., Woodward, J. G., Park-Sarge, O. K. & Sarge, K. D. Lowered temperature set point for activation of the cellular stress response in T-lymphocytes. J. Biol. Chem. 278, 9322–9326 (2003)
Jolly, C., Usson, Y. & Morimoto, R. I. Rapid and reversible relocalization of heat shock factor 1 within seconds to nuclear stress granules. Proc. Natl, Acad. Sci. USA 96, 6769–6774 (1999)
Panniers, R. Translational control during heat shock. Biochimie 76, 737–747 (1994)
Welch, W. J. & Suhan, J. P. Morphological study of the mammalian stress response: characterization of changes in cytoplasmic organelles, cytoskeleton, and nucleoli, and appearance of intranuclear actin filaments in rat fibroblasts after heat-shock treatment. J. Cell Biol. 101, 1198–1211 (1985)
Kristensen, P., Lund, A., Clark, B. F., Cavallius, J. & Merrick, W. C. Purification and characterisation of a tissue specific elongation factor 1 alpha (EF-1 α2) from rabbit muscle. Biochem. Biophys. Res. Commun. 245, 810–814 (1998)
Baler, R., Zou, J. & Voellmy, R. Evidence for a role of Hsp70 in the regulation of the heat shock response in mammalian cells. Cell Stress Chaperones 1, 33–39 (1996)
Farkas, T., Kutskova, Y. A. & Zimarino, V. Intramolecular repression of mouse heat shock factor 1. Mol. Cell. Biol. 18, 906–918 (1998)
Zhong, M., Orosz, A. & Wu, C. Direct sensing of heat and oxidation by Drosophila heat shock transcription factor. Mol. Cell 2, 101–108 (1998)
Zhong, M., Kim, S. J. & Wu, C. Sensitivity of Drosophila heat shock transcription factor to low pH. J. Biol. Chem. 274, 3135–3140 (1999)
Clos, J. et al. Molecular cloning and expression of a hexameric Drosophila heat shock factor subject to negative regulation. Cell 63, 1085–1097 (1990)
Szymanski, M. & Barciszewski, J. Regulation by RNA. Int. Rev. Cytol. 231, 197–258 (2003)
Nudler, E. & Mironov, A. S. The riboswitch control of bacterial metabolism. Trends Biochem. Sci. 29, 11–17 (2004)
Storz, G. An RNA thermometer. Genes Dev. 13, 633–636 (1999)
Westerheide, S. D. & Morimoto, R. I. Heat shock response modulators as therapeutic tools for diseases of protein conformation. J. Biol. Chem. 280, 33097–33100 (2005)
Acknowledgements
We thank M. Schick and E. Avetissova for technical assistance and N. J. Cowan for critical reading of the manuscript. This work was supported by grants from the NIH and the Edward Mallinckrodt Jr Foundation (E.N.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
Supplementary information
Supplementary Notes
This file contains the Supplementary Figures, Supplementary Methods, Supplementary Notes and additional references. (PDF 563 kb)
Rights and permissions
About this article
Cite this article
Shamovsky, I., Ivannikov, M., Kandel, E. et al. RNA-mediated response to heat shock in mammalian cells. Nature 440, 556–560 (2006). https://doi.org/10.1038/nature04518
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature04518
This article is cited by
-
Decoding the dual recognition mechanism of the glucocorticoid receptor for DNA and RNA: sequence versus shape
Scientific Reports (2023)
-
Genome-wide identification and functional prediction of long non-coding RNAs in Sprague-Dawley rats during heat stress
BMC Genomics (2021)
-
Third-Generation Sequencing Indicated that LncRNA Could Regulate eIF2D to Enhance Protein Translation Under Heat Stress in Populus simonii
Plant Molecular Biology Reporter (2021)
-
Genome-wide identification and characterization of long non-coding RNAs related to grain yield in foxtail millet [Setaria italica (L.) P. Beauv.]
BMC Genomics (2020)
-
Genome-wide identification of Arabidopsis long noncoding RNAs in response to the blue light
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.