Abstract
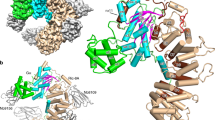
Epac proteins (exchange proteins directly activated by cAMP) are guanine-nucleotide-exchange factors (GEFs) for the small GTP-binding proteins Rap1 and Rap2 that are directly regulated by the second messenger cyclic AMP1,2 and function in the control of diverse cellular processes, including cell adhesion and insulin secretion3. Here we report the three-dimensional structure of full-length Epac2, a 110-kDa protein that contains an amino-terminal regulatory region with two cyclic-nucleotide-binding domains and a carboxy-terminal catalytic region. The structure was solved in the absence of cAMP and shows the auto-inhibited state of Epac. The regulatory region is positioned with respect to the catalytic region by a rigid, tripartite β-sheet-like structure we refer to as the ‘switchboard’ and an ionic interaction we call the ‘ionic latch’. As a consequence of this arrangement, the access of Rap to the catalytic site is sterically blocked. Mutational analysis suggests a model for cAMP-induced Epac activation with rigid body movement of the regulatory region, the features of which are universally conserved in cAMP-regulated proteins.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
de Rooij, J. et al. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature 396, 474–477 (1998)
Kawasaki, H. et al. A family of cAMP-binding proteins that directly activate Rap1. Science 282, 2275–2279 (1998)
Bos, J. L. Epac: a new cAMP target and new avenues in cAMP research. Nature Rev. Mol. Cell Biol. 4, 733–738 (2003)
de Rooij, J. et al. Mechanism of regulation of the Epac family of cAMP-dependent RapGEFs. J. Biol. Chem. 275, 20829–20836 (2000)
Boriack-Sjodin, P. A., Margarit, S. M., Bar-Sagi, D. & Kuriyan, J. The structural basis of the activation of Ras by Sos. Nature 394, 337–343 (1998)
Margarit, S. M. et al. Structural evidence for feedback activation by Ras·GTP of the Ras- specific nucleotide exchange factor SOS. Cell 112, 685–695 (2003)
Herrmann, C. Ras-effector interactions: after one decade. Curr. Opin. Struct. Biol. 13, 122–129 (2003)
Vijay-Kumar, S., Bugg, C. E. & Cook, W. J. Structure of ubiquitin refined at 1.8 Å resolution. J. Mol. Biol. 194, 531–544 (1987)
Huang, L., Hofer, F., Martin, G. S. & Kim, S. H. Structural basis for the interaction of Ras with RalGDS. Nature Struct. Biol. 5, 422–426 (1998)
Kabsch, W. & Sander, C. Dictionary of protein secondary structure: pattern recognition of hydrogen-bonded and geometrical features. Biopolymers 22, 2577–2637 (1983)
Liao, Y. et al. RA-GEF, a novel Rap1A guanine nucleotide exchange factor containing a Ras/Rap1A-associating domain, is conserved between nematode and humans. J. Biol. Chem. 274, 37815–37820 (1999)
Rebhun, J. F., Castro, A. F. & Quilliam, L. A. Identification of guanine nucleotide exchange factors (GEFs) for the Rap1 GTPase. Regulation of MR-GEF by M-Ras-GTP interaction. J. Biol. Chem. 275, 34901–34908 (2000)
Rehmann, H. et al. Structure and regulation of the cAMP-binding domains of Epac2. Nature Struct. Biol. 10, 26–32 (2003)
Rehmann, H., Rueppel, A., Bos, J. L. & Wittinghofer, A. Communication between the regulatory and the catalytic region of the cAMP-responsive guanine nucleotide exchange factor Epac. J. Biol. Chem. 278, 23508–23514 (2003)
Clayton, G. M., Silverman, W. R., Heginbotham, L. & Morais-Cabral, J. H. Structural basis of ligand activation in a cyclic nucleotide regulated potassium channel. Cell 119, 615–627 (2004)
Su, Y. et al. Regulatory subunit of protein kinase A: structure of deletion mutant with cAMP binding domains. Science 269, 807–813 (1995)
Kim, C., Xuong, N. H. & Taylor, S. S. Crystal structure of a complex between the catalytic and regulatory (RIα) subunits of PKA. Science 307, 690–696 (2005)
Rehmann, H., Schwede, F., Doskeland, S. O., Wittinghofer, A. & Bos, J. L. Ligand-mediated activation of the cAMP-responsive guanine nucleotide exchange factor Epac. J. Biol. Chem. 278, 38548–38556 (2003)
Canaves, J. M. & Taylor, S. S. Classification and phylogenetic analysis of the cAMP-dependent protein kinase regulatory subunit family. J. Mol. Evol. 54, 17–19 (2002)
Zagotta, W. N. et al. Structural basis for modulation and agonist specificity of HCN pacemaker channels. Nature 425, 200–205 (2003)
Acknowledgements
We thank T. Sixma for access to crystallization robots. We thank M. Weyand and A. Scrima for data collection and discussions. H.R. was supported by the Chemical Sciences of the Netherlands Organization for Scientific Research (NWO-CW) and is a recipient of the Otto-Hahn-Medaille of the Max-Planck-Gesellschaft. J.D. was supported by the Dutch Cancer Society.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Structure coordinates for Epac2 have been deposited in the Protein Data Bank under accession number 2BYV. Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
Supplementary information
Supplementary Notes
This file contains Supplementary Figures 1–5, Supplementary Figure Legends 1–5, Supplementary Table 1, Supplementary Methods and additional references. (PDF 2184 kb)
Rights and permissions
About this article
Cite this article
Rehmann, H., Das, J., Knipscheer, P. et al. Structure of the cyclic-AMP-responsive exchange factor Epac2 in its auto-inhibited state. Nature 439, 625–628 (2006). https://doi.org/10.1038/nature04468
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature04468
This article is cited by
-
Cyclic AMP induces reversible EPAC1 condensates that regulate histone transcription
Nature Communications (2023)
-
Membranes prime the RapGEF EPAC1 to transduce cAMP signaling
Nature Communications (2023)
-
Sensitive genetically encoded sensors for population and subcellular imaging of cAMP in vivo
Nature Methods (2022)
-
Modeling Epac1 interactions with the allosteric inhibitor AM-001 by co-solvent molecular dynamics
Journal of Computer-Aided Molecular Design (2020)
-
The Popeye domain containing gene family encoding a family of cAMP-effector proteins with important functions in striated muscle and beyond
Journal of Muscle Research and Cell Motility (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.