Abstract
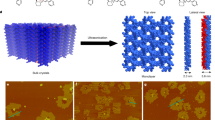
The concept of chirality dates back to 1848, when Pasteur manually separated left-handed from right-handed sodium ammonium tartrate crystals1. Crystallization is still an important means for separating chiral molecules into their two different mirror-image isomers (enantiomers)2, yet remains poorly understood3. For example, there are no firm rules to predict whether a particular pair of chiral partners will follow the behaviour of the vast majority of chiral molecules and crystallize together as racemic crystals4, or as separate enantiomers. A somewhat simpler and more tractable version of this phenomenon is crystallization in two dimensions, such as the formation of surface structures by adsorbed molecules. The relatively simple spatial molecular arrangement of these systems makes it easier to study the effects of specific chiral interactions5; moreover, chiral assembly and recognition processes can be observed directly and with molecular resolution using scanning tunnelling microscopy6,7,8,9. The enantioseparation of chiral molecules in two dimensions is expected to occur more readily because planar confinement excludes some bulk crystal symmetry elements and enhances chiral interactions10,11; however, many surface structures have been found to be racemic12,13,14,15,16,17,18. Here we show that the chiral hydrocarbon heptahelicene on a Cu(111) surface does not undergo two-dimensional spontaneous resolution into enantiomers19, but still shows enantiomorphism on a mesoscopic length scale that is readily amplified. That is, we observe formation of racemic heptahelicene domains with non-superimposable mirror-like lattice structures, with a small excess of one of the heptahelicene enantiomers suppressing the formation of one domain type. Similar to the induction of homochirality in achiral enantiomorphous monolayers20 by a chiral modifier, a small enantiomeric excess suffices to ensure that the entire molecular monolayer consists of domains having only one of two possible, non-superimposable, mirror-like lattice structures.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Pasteur, L. Recherches sur les relations qui peuvent exister entre la forme crystalline et la composition chimique, et le sens de la polarisation rotatoire. Ann. Chim. Phys. 24, 442–459 (1848)
Sheldon, R. A. in Chiral Technologies: Industrial Synthesis of Optically Active Compounds 173–204 (M. Dekker, New York, 1993)
Addadi, L. & Weiner, S. Crystals, asymmetry and life. Nature 411, 753–754 (2001)
Jacques, J., Collet, A. & Wilen, S. H. in Enantiomers, Racemates and Resolution (Wiley, New York, 1981)
Maddox, J. Racemization rationalized. Nature 341, 101 (1989)
De Feyter, S. & De Schryver, F. C. Two-dimensional supramolecular self-assembly probed by scanning tunneling microscopy. Chem. Soc. Rev. 32, 139–150 (2003)
Ortega Lorenzo, M., Baddeley, C. J., Muryn, C. & Raval, R. Extended surface chirality from supramolecular assemblies of adsorbed chiral molecules. Nature 404, 376–379 (2000)
Kühnle, A., Linderoth, T. R., Hammer, B. & Besenbacher, F. Chiral recognition in dimerization of adsorbed cysteine observed by scanning tunnelling microscopy. Nature 415, 891–893 (2002)
Chen, Q. & Richardson, N. V. Enantiomeric interactions between nucleic acid bases and amino acids on solid surfaces. Nature Mater. 2, 324–328 (2003)
Kuzmenko, I. et al. Aspects of spontaneous separation of enantiomers in two- and three-dimensional crystals. Chirality 10, 415–424 (1998)
Perez-Garcia, L. & Amabilino, D. B. Spontaneous resolution under supramolecular control. Chem. Soc. Rev. 31, 342–356 (2002)
De Feyter, S. et al. Homo- and heterochiral supramolecular tapes from achiral, enantiopure, and racemic promesogenic formamides. Angew. Chem. Int. Edn Engl. 40, 3217–3220 (2001)
Romer, S., Behzadi, B., Fasel, R. & Ernst, K.-H. Homochiral conglomerates and racemic crystals in two dimensions: Tartaric acid on Cu(110). Chem. Eur. J. 11, 4149–4154 (2005)
Cai, Y. & Bernasek, S. L. Chiral pair monolayer adsorption of iodine-substituted octadecanol molecules on graphite. J. Am. Chem. Soc. 125, 1655–1659 (2003)
Wie, Y., Kannappan, K., Flynn, G. W. & Zimmt, M. B. Scanning tunneling microscopy of prochiral anthracene derivatives on graphite: chain length effects on monolayer morphology. J. Am. Chem. Soc. 126, 5318–5322 (2004)
Vidal, F. et al. Chiral phase transition in two-dimensional supramolecular assemblies of prochiral molecules. J. Am. Chem. Soc. 127, 10101–10106 (2005)
Cai, Y. & Bernasek, S. L. Structures formed by the chiral assembly of racemic mixtures of enantiomers: iodination products of elaidic and oleic acids. J. Phys. Chem. B 109, 4514–4519 (2005)
Böhringer, M., Schneider, W.-D. & Berndt, R. Real space observation of a chiral phase transition in a two-dimensional organic layer. Angew. Chem. Int. Edn Engl. 39, 792–795 (2000)
Ernst, K.-H., Kuster, Y., Fasel, R., Müller, M. & Ellerbeck, U. Two-dimensional separation of [7]helicene enantiomers on Cu(111). Chirality 13, 675–678 (2001)
Parschau, M., Romer, S. & Ernst, K.-H. Induction of homochirality in achiral enantiomorphous monolayers. J. Am. Chem. Soc. 126, 15398–15399 (2004)
Fasel, R., Parschau, M. & Ernst, K.-H. Chirality transfer from single molecules into self-assembled monolayers. Angew. Chem. Int. Edn Engl. 42, 5178–5181 (2003)
Barlow, S. M. & Raval, R. Complex molecules at metal surfaces: bonding, organisation and chirality. Surf. Sci. Rep. 50, 201–341 (2003)
Green, M. M. et al. The macromolecular route to chiral amplification. Angew. Chem. Int. Edn Engl. 38, 3138–3154 (1999)
Acknowledgements
This work was supported by the Swiss National Science Foundation (SNF). We thank O. Gröning for writing the software to simulate STM images and U. Ellerbeck for the [7]H synthesis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
Supplementary information
Supplementary Methods
This file describes the experimental procedures and gives detail on the molecular mechanics calculations. (PDF 149 kb)
Supplementary Figure 1
This figure describes the segregation of enantiomeric excess to steps and into residual areas. (PDF 124 kb)
Supplementary Figure 2
This figure describes the calculated energy cost when single M- or P- heptahelicene molecules in λ and ρ domains are substituted by opposite enantiomers. (PDF 205 kb)
Supplementary Figure 3
This figure describes the higher energy content of mirror domain boundaries with respect to rotational domain boundaries. (PDF 453 kb)
Supplementary Figure 4
This figure describes the calculation of the energy at the interface between a l domain edge and excess molecules of different handedness. (PDF 156 kb)
Rights and permissions
About this article
Cite this article
Fasel, R., Parschau, M. & Ernst, KH. Amplification of chirality in two-dimensional enantiomorphous lattices. Nature 439, 449–452 (2006). https://doi.org/10.1038/nature04419
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature04419
This article is cited by
-
Kinetic control over the chiral-selectivity in the formation of organometallic polymers on a Ag(110) surface
Communications Chemistry (2024)
-
Chirality control of a single carbene molecule by tip-induced van der Waals interactions
Nature Communications (2023)
-
Condensation and asymmetric amplification of chirality in achiral molecules adsorbed on an achiral surface
Nature Communications (2023)
-
Evolution of Br⋯Br contacts in enantioselective molecular recognition during chiral 2D crystallization
Nature Communications (2022)
-
Controlling anisotropic properties by manipulating the orientation of chiral small molecules
Nature Chemistry (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.