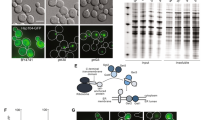
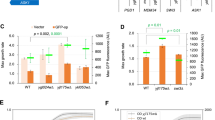
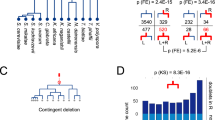
Abstract
Yeast genetics and in vitro biochemical analysis have identified numerous genes involved in protein secretion1,2. As compared with yeast, however, the metazoan secretory pathway is more complex and many mechanisms that regulate organization of the Golgi apparatus remain poorly characterized. We performed a genome-wide RNA-mediated interference screen in a Drosophila cell line to identify genes required for constitutive protein secretion. We then classified the genes on the basis of the effect of their depletion on organization of the Golgi membranes. Here we show that depletion of class A genes redistributes Golgi membranes into the endoplasmic reticulum, depletion of class B genes leads to Golgi fragmentation, depletion of class C genes leads to aggregation of Golgi membranes, and depletion of class D genes causes no obvious change. Of the 20 new gene products characterized so far, several localize to the Golgi membranes and the endoplasmic reticulum.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Novick, P. & Schekman, R. Secretion and cell-surface growth are blocked in a temperature-sensitive mutant of Saccharomyces cerevisiae. Proc. Natl Acad. Sci. USA 76, 1858–1862 (1979)
Rothman, J. E. Mechanisms of intracellular protein transport. Nature 372, 55–63 (1994)
Nichols, B. J. & Pelham, H. R. SNAREs and membrane fusion in the Golgi apparatus. Biochim. Biophys. Acta 1404, 9–31 (1998)
Duden, R., Allan, V. & Kreis, T. Involvement of β-COP in membrane traffic through the Golgi complex. Trends Cell Biol. 1, 14–19 (1991)
Serafini, T. et al. A coat subunit of Golgi-derived non-clathrin-coated vesicles with homology to the clathrin-coated vesicle coat protein β-adaptin. Nature 349, 215–220 (1991)
Boutros, M. et al. Genome-wide RNAi analysis of growth and viability in Drosophila cells. Science 303, 832–835 (2004)
DasGupta, R., Kaykas, A., Moon, R. T. & Perrimon, N. Functional genomic analysis of the Wnt-wingless signalling pathway. Science 308, 826–833 (2005)
Eggert, U. S. et al. Parallel chemical genetic and genome-wide RNAi screens identify cytokinesis inhibitors and targets. PLoS Biol. 2, e379 (2004)
Kiger, A. A. et al. A functional genomic analysis of cell morphology using RNA interference. J. Biol. 2, 27 (2003)
Stanley, H., Botas, J. & Malhotra, V. The mechanism of Golgi segregation during mitosis is cell type-specific. Proc. Natl Acad. Sci. USA 94, 14467–14470 (1997)
Shorter, J. & Warren, G. Golgi architecture and inheritance. Annu. Rev. Cell. Dev. Biol. 18, 379–420 (2002)
Uhlmann, F. Separase regulation during mitosis. Biochem. Soc. Symp. 70, 243–251 (2003)
Sharp, D. J. Cell division: MAST sails through mitosis. Curr. Biol. 12, R585–R587 (2002)
Snaith, H. A., Armstrong, C. G., Guo, Y., Kaiser, K. & Cohen, P. T. Deficiency of protein phosphatase 2A uncouples the nuclear and centrosome cycles and prevents attachment of microtubules to the kinetochore in Drosophila microtubule star (mts) embryos. J. Cell Sci. 109, 3001–3012 (1996)
Dilcher, M. et al. Use1p is a yeast SNARE protein required for retrograde traffic to the ER. EMBO J. 22, 3664–3674 (2003)
Chua, J. J., Ng, M. M. & Chow, V. T. The non-structural 3 (NS3) protein of dengue virus type 2 interacts with human nuclear receptor binding protein and is associated with alterations in membrane structure. Virus Res. 102, 151–163 (2004)
De Langhe, S., Haataja, L., Senadheera, D., Groffen, J. & Heisterkamp, N. Interaction of the small GTPase Rac3 with NRBP, a protein with a kinase-homology domain. Int. J. Mol. Med. 9, 451–459 (2002)
Connolly, C. N., Futter, C. E., Gibson, A., Hopkins, C. R. & Cutler, D. F. Transport into and out of the Golgi complex studied by transfecting cells with cDNAs encoding horseradish peroxidase. J. Cell Biol. 127, 641–652 (1994)
Acknowledgements
We thank members of the Malhotra laboratory for discussions; members of the DRSC for advice; the Institute for Chemistry and Cell Biology for use of their Cybio robot; and J. Feramisco and members of the UCSD Cancer Center imaging facility for help with microscopy. Work in the Malhotra laboratory is supported by NIH grants and a senior investigator award from Sandler's Program for Asthma Research. N.P. is a Howard Hughes investigator.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
Supplementary information
Supplementary Figure 1
Golgi fragmentation and block in HRP secretion are not due to an arrest in mitosis. (PDF 541 kb)
Supplementary Table 1
1133 dsRNAs inducing a significant inhibition of HRP secretion. (PDF 97 kb)
Supplementary Table 2
154 dsRNAs tested in secondary screens and reasons for their removal. (PDF 36 kb)
Supplementary Table 3
130 genes involved in secretion and Golgi membrane organization. (PDF 32 kb)
Supplementary Legends
Text to accompany the above Supplementary Figure and Supplementary Tables. (DOC 40 kb)
Rights and permissions
About this article
Cite this article
Bard, F., Casano, L., Mallabiabarrena, A. et al. Functional genomics reveals genes involved in protein secretion and Golgi organization. Nature 439, 604–607 (2006). https://doi.org/10.1038/nature04377
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature04377
This article is cited by
-
In vivo identification of Drosophila rhodopsin interaction partners by biotin proximity labeling
Scientific Reports (2024)
-
HRG-9 homologues regulate haem trafficking from haem-enriched compartments
Nature (2022)
-
The genome trilogy of Anopheles stephensi, an urban malaria vector, reveals structure of a locus associated with adaptation to environmental heterogeneity
Scientific Reports (2022)
-
A protein that mobilizes the cofactor molecule haem for use in cells
Nature (2022)
-
Mitochondrial dysfunction associated with TANGO2 deficiency
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.