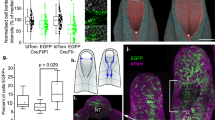
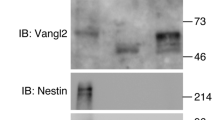
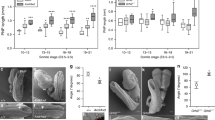
Abstract
Environmental and genetic aberrations lead to neural tube closure defects (NTDs) in 1 out of every 1,000 births1. Mouse and frog models for these birth defects have indicated that Van Gogh-like 2 (Vangl2, also known as Strabismus) and other components of planar cell polarity (PCP) signalling might control neurulation by promoting the convergence of neural progenitors to the midline2,3,4,5,6,7,8. Here we show a novel role for PCP signalling during neurulation in zebrafish. We demonstrate that non-canonical Wnt/PCP signalling polarizes neural progenitors along the anteroposterior axis. This polarity is transiently lost during cell division in the neural keel but is re-established as daughter cells reintegrate into the neuroepithelium. Loss of zebrafish Vangl2 (in trilobite mutants) abolishes the polarization of neural keel cells, disrupts re-intercalation of daughter cells into the neuroepithelium, and results in ectopic neural progenitor accumulations and NTDs. Remarkably, blocking cell division leads to rescue of trilobite neural tube morphogenesis despite persistent defects in convergence and extension. These results reveal a function for PCP signalling in coupling cell division and morphogenesis at neurulation and indicate a previously unrecognized mechanism that might underlie NTDs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Copp, A. J., Greene, N. D. & Murdoch, J. N. The genetic basis of mammalian neurulation. Nature Rev. Genet. 4, 784–793 (2003)
Greene, N. D., Gerrelli, D., Van Straaten, H. W. & Copp, A. J. Abnormalities of floor plate, notochord and somite differentiation in the loop-tail (Lp) mouse: a model of severe neural tube defects. Mech. Dev. 73, 59–72 (1998)
Kibar, Z. et al. Ltap, a mammalian homolog of Drosophila Strabismus/Van Gogh, is altered in the mouse neural tube mutant Loop-tail. Nature Genet. 28, 251–255 (2001)
Murdoch, J. N., Doudney, K., Paternotte, C., Copp, A. J. & Stanier, P. Severe neural tube defects in the loop-tail mouse result from mutation of Lpp1, a novel gene involved in floor plate specification. Hum. Mol. Genet. 10, 2593–2601 (2001)
Keller, R. Shaping the vertebrate body plan by polarized embryonic cell movements. Science 298, 1950–1954 (2002)
Wallingford, J. B., Fraser, S. E. & Harland, R. M. Convergent extension: the molecular control of polarized cell movement during embryonic development. Dev. Cell 2, 695–706 (2002)
Goto, T. & Keller, R. The planar cell polarity gene strabismus regulates convergence and extension and neural fold closure in Xenopus. Dev. Biol. 247, 165–181 (2002)
Wallingford, J. B. & Harland, R. M. Neural tube closure requires Dishevelled- dependent convergent extension of the midline. Development 129, 5815–5825 (2002)
Kimmel, C. B., Warga, R. M. & Kane, D. A. Cell cycles and clonal strings during formation of the zebrafish central nervous system. Development 120, 265–276 (1994)
Concha, M. L. & Adams, R. J. Oriented cell divisions and cellular morphogenesis in the zebrafish gastrula and neurula: a time-lapse analysis. Development 125, 983–994 (1998)
Geldmacher-Voss, B., Reugels, A. M., Pauls, S. & Campos-Ortega, J. A. A 90-degree rotation of the mitotic spindle changes the orientation of mitoses of zebrafish neuroepithelial cells. Development 130, 3767–3780 (2003)
Park, M. & Moon, R. T. The planar cell-polarity gene stbm regulates cell behaviour and cell fate in vertebrate embryos. Nature Cell Biol. 4, 20–25 (2002)
Jessen, J. R. et al. Zebrafish trilobite identifies new roles for Strabismus in gastrulation and neuronal movements. Nature Cell Biol. 4, 610–615 (2002)
Ciruna, B. et al. Production of maternal-zygotic mutant zebrafish by germ-line replacement. Proc. Natl Acad. Sci. USA 99, 14919–14924 (2002)
Heisenberg, C. P. et al. Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature 405, 76–81 (2000)
Rauch, G. J. et al. Wnt5 is required for tail formation in the zebrafish embryo. Cold Spring Harb. Symp. Quant. Biol. 62, 227–234 (1997)
Matsui, T. et al. Noncanonical Wnt signaling regulates midline convergence of organ primordia during zebrafish development. Genes Dev. 19, 164–175 (2005)
Wallingford, J. B. & Harland, R. M. Xenopus Dishevelled signaling regulates both neural and mesodermal convergent extension: parallel forces elongating the body axis. Development 128, 2581–2592 (2001)
Thisse, C. & Thisse, B. Antivin, a novel and divergent member of the TGFβ superfamily, negatively regulates mesoderm induction. Development 126, 229–240 (1999)
Meno, C. et al. Mouse Lefty2 and zebrafish antivin are feedback inhibitors of nodal signaling during vertebrate gastrulation. Mol. Cell 4, 287–298 (1999)
Ando, R., Hama, H., Yamamoto-Hino, M., Mizuno, H. & Miyawaki, A. An optical marker based on the UV-induced green-to-red photoconversion of a fluorescent protein. Proc. Natl Acad. Sci. USA 99, 12651–12656 (2002)
Strutt, D. I. The asymmetric subcellular localisation of components of the planar polarity pathway. Semin. Cell Dev. Biol. 13, 225–231 (2002)
Jiang, D., Munro, E. M. & Smith, W. C. Ascidian prickle regulates both mediolateral and anterior–posterior cell polarity of notochord cells. Curr. Biol. 15, 79–85 (2005)
Jenny, A., Darken, R. S., Wilson, P. A. & Mlodzik, M. Prickle and Strabismus form a functional complex to generate a correct axis during planar cell polarity signaling. EMBO J. 22, 4409–4420 (2003)
Lyons, D. A. et al. erbb3 and erbb2 are essential for Schwann cell migration and myelination in zebrafish. Curr. Biol. 15, 513–524 (2005)
Gong, Y., Mo, C. & Fraser, S. E. Planar cell polarity signalling controls cell division orientation during zebrafish gastrulation. Nature 430, 689–693 (2004)
Djiane, A., Yogev, S. & Mlodzik, M. The apical determinants aPKC and dPatj regulate Frizzled-dependent planar cell polarity in the Drosophila eye. Cell 121, 621–631 (2005)
van Straaten, H. W. & Copp, A. J. Curly tail: a 50-year history of the mouse spina bifida model. Anat. Embryol. (Berl.) 203, 225–237 (2001)
Gritsman, K. et al. The EGF-CFC protein one-eyed pinhead is essential for nodal signaling. Cell 97, 121–132 (1999)
Carreira-Barbosa, F. et al. Prickle 1 regulates cell movements during gastrulation and neuronal migration in zebrafish. Development 130, 4037–4046 (2003)
Acknowledgements
We thank W. Talbot and D. Lyons for sharing their protocol for pharmacological inhibition of cell division, A. Chitnis for useful discussion, and L. Solnica-Krezel, W. Talbot, J. Wallingford, A. Giraldez, D. Prober and J. Rihel for comments on the manuscript. This work was supported by grants from the NIH to A.F.S. and M.M. A.F.S. was a Scholar of the McKnight Foundation for Neuroscience, an Irma T. Hirschl Trust Career Scientist and an Established Investigator of the American Heart Association. B.C. was supported by a long-term fellowship from the Human Frontier Science Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
Supplementary information
Supplementary Figure 1
This figure shows transverse sections taken through the anterior trunk of fixed wild-type embryos, ranging from 5-somite to 20-somite stages. The figure illustrates the morphogenesis of the neural plate into a neural tube. (DOC 259 kb)
Supplementary Figure 2
This figure shows lateral and dorsal views of wild-type, zygotic trilobite, and maternal-zygotic trilobite mutant embryos at 24 hpf. (DOC 125 kb)
Supplementary Figure 3
This figure depicts the strategy used to generate silberblick;pipetail germ line-replacement chimeras. (DOC 175 kb)
Supplementary Figure 4
This figure describes the ppt(sk13) allele used in our studies, and shows MZslb;MZppt and MZslb;MZppt+wnt4MO mutant phenotypes at 30hpf. (DOC 218 kb)
Supplementary Figure 5
This figure shows dorsal views of the neural keel of wild-type and MZtri mutant embryos, and demonstrates ectopic accumulation of MZtri neural progenitor cells. (DOC 165 kb)
Supplementary Figure 6
This figure shows rescue of the zebrafish Pk1 morphant phenotype through injection of Gfp-Pk mRNA. (DOC 139 kb)
Supplementary Figure 7
This figure shows images taken from time-lapse movies of neuroepithelial cell behaviour in the neural keel of genetically chimeric embryos, and demonstrates both cell autonomy and non-autonomy of Vangl2 function. (DOC 571 kb)
Supplementary Figure 8
This figure shows the effects of blocking cell division on wild-type and MZtri embryonic development. Lateral and dorsal views of live embryos are shown, as well as transverse sections through the anterior trunk of shh-stained embryos. (DOC 241 kb)
Supplementary Table 1
This table summarizes the results of WT and MZtri transplantation studies into the autonomy of Vangl2 function in regulating the intercalation of neural progenitor cells into the contralateral neuroepithelial layer following mitosis. (DOC 42 kb)
Supplementary Video 1
This movie shows cell division in the neural keel of a WT embryo that has been scatter labeled for mGFP. The confocal time lapse covers a period of 21 min, and demonstrates the rapid intercalation the apical neuroepithelial daughter cell into the contralateral neuroepithelial layer following mitosis. (MOV 3480 kb)
Supplementary Video 2
This movie shows cell division in the forming neural keel of an MZtri embryo that has been scatter labeled for mGFP. The confocal time lapse covers a period of 53 min, and demonstrates that MZtri neural progenitors fail to re-intercalate into the neuroepithelium following mitosis. (MOV 9014 kb)
Supplementary Video 3
This movie shows the neural keel of a WT embryo that has been scatter labeled for Gfp-Pk. The confocal time lapse covers a period of 30 min, and demonstrates the dynamic punctate localization of Gfp-Pk along the anterior membrane of neural progenitor cells. (MOV 3369 kb)
Rights and permissions
About this article
Cite this article
Ciruna, B., Jenny, A., Lee, D. et al. Planar cell polarity signalling couples cell division and morphogenesis during neurulation. Nature 439, 220–224 (2006). https://doi.org/10.1038/nature04375
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature04375
This article is cited by
-
The role of prickle proteins in vertebrate development and pathology
Molecular and Cellular Biochemistry (2023)
-
Live imaging and conditional disruption of native PCP activity using endogenously tagged zebrafish sfGFP-Vangl2
Nature Communications (2022)
-
Apical–basal polarity and the control of epithelial form and function
Nature Reviews Molecular Cell Biology (2022)
-
Wnt/planar cell polarity signaling controls morphogenetic movements of gastrulation and neural tube closure
Cellular and Molecular Life Sciences (2022)
-
Planar cell polarity pathway in kidney development, function and disease
Nature Reviews Nephrology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.