Abstract
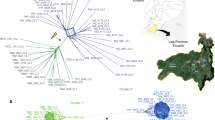
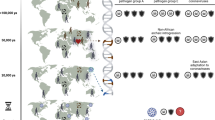
Genealogy can illuminate the evolutionary path of important human pathogens. In some microbes, strict clonal reproduction predominates, as with the worldwide dissemination of Mycobacterium leprae, the cause of leprosy1. In other pathogens, sexual reproduction yields clones with novel attributes, for example, enabling the efficient, oral transmission of the parasite Toxoplasma gondii2. However, the roles of clonal or sexual propagation in the origins of many other microbial pathogen outbreaks remain unknown, like the recent fungal meningoencephalitis outbreak on Vancouver Island, Canada, caused by Cryptococcus gattii3. Here we show that the C. gattii outbreak isolates comprise two distinct genotypes. The majority of isolates are hypervirulent and have an identical genotype that is unique to the Pacific Northwest. A minority of the isolates are significantly less virulent and share an identical genotype with fertile isolates from an Australian recombining population. Genotypic analysis reveals evidence of sexual reproduction, in which the majority genotype is the predicted offspring. However, instead of the classic a–α sexual cycle, the majority outbreak clone appears to have descended from two α mating-type parents. Analysis of nuclear content revealed a diploid environmental isolate homozygous for the major genotype, an intermediate produced during same-sex mating. These studies demonstrate how cryptic same-sex reproduction can enable expansion of a human pathogen to a new geographical niche and contribute to the ongoing production of infectious spores. This has implications for the emergence of other microbial pathogens and inbreeding in host range expansion in the fungal and other kingdoms.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Monot, M. et al. On the origin of leprosy. Science 308, 1040–1042 (2005)
Su, C. et al. Recent expansion of Toxoplasma through enhanced oral transmission. Science 299, 414–416 (2003)
Hoang, L. M., Maguire, J. A., Doyle, P., Fyfe, M. & Roscoe, D. L. Cryptococcus neoformans infections at Vancouver Hospital and Health Sciences Centre (1997–2002): epidemiology, microbiology and histopathology. J. Med. Microbiol. 53, 935–940 (2004)
Casadevall, A. & Perfect, J. R. Cryptococcus neoformans (ASM Press, Washington DC, 1998)
Kwon-Chung, K. J., Boekhout, T., Fell, J. W. & Diaz, M. Proposal to conserve the name Cryptococcus gattii against C. hondurianus and C. bacillisporus (Basidiomycota, Hymenomycetes, Tremellomycetiadae). Taxon 51, 804–806 (2002)
Ruiz, A. & Bulmer, G. S. Particle size of airborn Cryptococcus neoformans in a tower. Appl. Environ. Microbiol. 41, 1225–1229 (1981)
Sukroongreung, S., Kitiniyom, K., Nilakul, C. & Tantimavanich, S. Pathogenicity of basidiospores of Filobasidiella neoformans var. neoformans. Med. Mycol. 36, 419–424 (1998)
Hull, C. M. & Heitman, J. Genetics of Cryptococcus neoformans. Annu. Rev. Genet. 36, 557–615 (2002)
Fraser, J. A., Subaran, R. L., Nichols, C. B. & Heitman, J. Recapitulation of the sexual cycle of the primary fungal pathogen Cryptococcus neoformans var. gattii: implications for an outbreak on Vancouver Island, Canada. Eukaryot. Cell 2, 1036–1045 (2003)
Kidd, S. E. et al. A rare genotype of Cryptococcus gattii caused the cryptococcosis outbreak on Vancouver Island (British Columbia, Canada). Proc. Natl Acad. Sci. USA 101, 17258–17263 (2004)
Lin, X., Hull, C. M. & Heitman, J. Sexual reproduction between partners of the same mating type in Cryptococcus neoformans. Nature 434, 1017–1021 (2005)
Boekhout, T. et al. Hybrid genotypes in the pathogenic yeast Cryptococcus neoformans. Microbiol. 147, 891–907 (2001)
Litvintseva, A. P., Thakur, R., Vilgalys, R. & Mitchell, T. G. Multilocus sequence typing reveals three genetically distinct subpopulations of Cryptococcus neoformans var. grubii (serotype A), including a unique population in Botswana. Genetics (in the press)
Maiden, M. C. et al. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc. Natl Acad. Sci. USA 95, 3140–3145 (1998)
Hull, C. M., Boily, M. J. & Heitman, J. Sex-specific homeodomain proteins Sxi1α and Sxi2a coordinately regulate sexual development in Cryptococcus neoformans. Eukaryot. Cell 4, 526–535 (2005)
Fraser, J. A. et al. Convergent evolution of chromosomal sex-determining regions in the animal and fungal kingdoms. PLoS Biol. 2, 2243–2255 (2004)
Diaz, M. R., Boekhout, T., Theelen, B. & Fell, J. W. Molecular sequence analyses of the intergenic spacer (IGS) associated with rDNA of the two varieties of the pathogenic yeast, Cryptococcus neoformans. Syst. Appl. Microbiol. 23, 535–545 (2000)
Campbell, L. T. et al. Clonality and recombination in genetically differentiated subgroups of Cryptococcus gattii. Eukaryot. Cell 4, 1403–1409 (2005)
Campbell, L. T. et al. Clinical and environmental isolates of Cryptococcus gattii from Australia that retain sexual fecundity. Eukaryot. Cell 4, 1410–1419 (2005)
Halliday, C. L. & Carter, D. A. Clonal reproduction and limited dispersal in an environmental population of Cryptococcus neoformans var. gattii isolates from Australia. J. Clin. Microbiol. 41, 703–711 (2003)
Loftus, B. J. et al. The genome of the basidiomycetous yeast and human pathogen Cryptococcus neoformans. Science 307, 1321–1324 (2005)
Kwon-Chung, K. J. A new species of Filobasidiella, the sexual state of Cryptococcus neoformans B and C serotypes. Mycologia 68, 943–946 (1976)
Shimizu, K. K. et al. Darwinian selection on a selfing locus. Science 306, 2081–2084 (2004)
Wickes, B. L., Mayorga, M. E., Edman, U. & Edman, J. C. Dimorphism and haploid fruiting in Cryptococcus neoformans: association with the α-mating type. Proc. Natl Acad. Sci. USA 93, 7327–7331 (1996)
Grigg, M. E., Bonnefoy, S., Hehl, A. B., Suzuki, Y. & Boothroyd, J. C. Success and virulence in Toxoplasma as the result of sexual recombination between two distinct ancestries. Science 294, 161–165 (2001)
Thomas, C. F. Jr & Limper, A. H. Pneumocystis pneumonia. N. Engl. J. Med. 350, 2487–2498 (2004)
Gaunt, M. W. et al. Mechanism of genetic exchange in American trypanosomes. Nature 421, 936–939 (2003)
Alano, P. & Carter, R. Sexual differentiation in malaria parasites. Annu. Rev. Microbiol. 44, 429–449 (1990)
Thompson, J. D., Higgins, D. G. & Gibson, T. J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22, 4673–4680 (1994)
Cox, G. M. et al. Superoxide dismutase influences the virulence of Cryptococcus neoformans by affecting growth within macrophages. Infect. Immun. 71, 173–180 (2003)
Acknowledgements
We thank J. Kronstad for the R265 BAC library and strains; A. Litvintseva for MLST information; F. Dromer, D. Carter and C. Paula for strains; A. Idnurm, C. Fraser, X. Lin, K. Nielsen, J. Blankenship, A. Litvintseva, D. Lew and J. Anderson for reading the manuscript; and the Broad Fungal Genome Initiative. This work was supported by an NIAID R01 grant to J.H. Author Contributions J.A.F. and J.H. designed the experiments and wrote the manuscript. J.A.F. conducted or participated in all of the experiments, and S.S.G. and S.G.G-B. assisted with animal experiments. J.R.W. and J.R.P. supervised animal experiments and assisted with data analysis. E.C.W. contributed technical support. S.D. assisted with phylogenetic analysis. A.A. and F.S.D. contributed sequence analyses for MLST and MAT, and J.E.S. conducted computer modelling for genetic drift.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
DNA sequences identified in this study have been deposited in GenBank; a full list of accession numbers is available in Supplementary Table 5. Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
Supplementary information
Supplementary Figure S1
Congruence of gene phylogeny in C. gattii. (PDF 65 kb)
Supplementary Notes
This file contains Supplementary Methods and Supplementary Discussion. (DOC 58 kb)
Supplementary Table S1
A spreadsheet detailing strain MLST data (XLS 58 kb)
Supplementary Table S2
A spreadsheet detailing strain more MLST data (XLS 21 kb)
Supplementary Table S3
A spreadsheet detailing strain MAT locus fingerprint data (XLS 58 kb)
Supplementary Table S4
A spreadsheet listing the oligonucleotide primers employed in this study (XLS 34 kb)
Supplementary Table S5
A spreadsheet listing the GenBank accession numbers of DNA sequences described in this study (XLS 65 kb)
Rights and permissions
About this article
Cite this article
Fraser, J., Giles, S., Wenink, E. et al. Same-sex mating and the origin of the Vancouver Island Cryptococcus gattii outbreak. Nature 437, 1360–1364 (2005). https://doi.org/10.1038/nature04220
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature04220
This article is cited by
-
Emerging Antifungal Resistance in Fungal Pathogens
Current Clinical Microbiology Reports (2024)
-
Cryptococcal meningitis
Nature Reviews Disease Primers (2023)
-
Regulatory basis for reproductive flexibility in a meningitis-causing fungal pathogen
Nature Communications (2022)
-
Cryptococcus spp. and Cryptococcosis: focusing on the infection in Brazil
Brazilian Journal of Microbiology (2022)
-
Multilocus Sequence Typing of Clinical Isolates of Cryptococcus from India
Mycopathologia (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.