Abstract
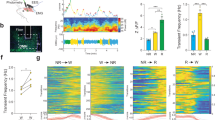
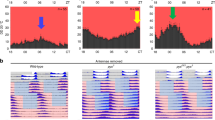
The biochemical machinery that underlies circadian rhythms is conserved among animal species and drives self-sustained molecular oscillations and functions, even within individual asynchronous tissue-culture cells1,2,3. Yet the rhythm-generating neural centres of higher eukaryotes are usually composed of interconnected cellular networks, which contribute to robustness and synchrony as well as other complex features of rhythmic behaviour4,5,6,7. In mammals, little is known about how individual brain oscillators are organized to orchestrate a complex behavioural pattern. Drosophila is arguably more advanced from this point of view: we and others have recently shown that a group of adult brain clock neurons expresses the neuropeptide PDF8 and controls morning activity (small LNv cells; M-cells), whereas another group of clock neurons controls evening activity (CRY+, PDF- cells; E-cells)6,9. We have generated transgenic mosaic animals with different circadian periods in morning and evening cells. Here we show, by behavioural and molecular assays, that the six canonical groups of clock neurons10 are organized into two separate neuronal circuits. One has no apparent effect on locomotor rhythmicity in darkness, but within the second circuit the molecular and behavioural timing of the evening cells is determined by morning-cell properties. This is due to a daily resetting signal from the morning to the evening cells, which run at their genetically programmed pace between consecutive signals. This neural circuit and oscillator-coupling mechanism ensures a proper relationship between the timing of morning and evening locomotor activity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Welsh, D. K., Yoo, S. H., Liu, A. C., Takahashi, J. S. & Kay, S. A. Bioluminescence imaging of individual fibroblasts reveals persistent, independently phased circadian rhythms of clock gene expression. Curr. Biol. 14, 2289–2295 (2004)
Balsalobre, A., Damiola, F. & Schibler, U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell 93, 929–937 (1998)
Nagoshi, E. et al. Circadian gene expression in individual fibroblasts: cell-autonomous and self-sustained oscillators pass time to daughter cells. Cell 119, 693–705 (2004)
Harmar, A. et al. The VPAC2 receptor is essential for circadian function in the mouse suprachiasmatic nuclei. Cell 109, 497–508 (2002)
Peng, Y., Stoleru, D., Levine, J. D., Hall, J. C. & Rosbash, M. Drosophila free-running rhythms require intercellular communication. PLoS Biol. 1, E13 (2003)
Stoleru, D., Peng, Y., Agosto, J. & Rosbash, M. Coupled oscillators control morning and evening locomotor behaviour of Drosophila. Nature 431, 862–868 (2004)
Lin, Y., Stormo, G. D. & Taghert, P. H. The neuropeptide pigment-dispersing factor coordinates pacemaker interactions in the Drosophila circadian system. J. Neurosci. 24, 7951–7957 (2004)
Renn, S. C., Park, J. H., Rosbash, M., Hall, J. C. & Taghert, P. H. A pdf neuropeptide gene mutation and ablation of PDF neurons each cause severe abnormalities of behavioural circadian rhythms in Drosophila. Cell 99, 791–802 (1999)
Grima, B., Chelot, E., Xia, R. & Rouyer, F. Morning and evening peaks of activity rely on different clock neurons of the Drosophila brain. Nature 431, 869–873 (2004)
Kaneko, M., Helfrich-Forster, C. & Hall, J. C. Spatial and temporal expression of the period and timeless genes in the developing nervous system of Drosophila: newly identified pacemakers candidates and novel features of clock gene product cycling. J. Neurosci. 17, 6745–6760 (1997)
Martinek, S., Inonog, S., Manoukian, A. S. & Young, M. W. A role for the segment polarity gene shaggy/GSK-3 in the Drosophila circadian clock. Cell 105, 769–779 (2001)
Kaneko, M. & Hall, J. C. Neuroanatomy of cells expressing clock genes in Drosophila: Transgenic manipulation of the period and timeless genes to mark the perikarya of circadian pacemaker neurons and their projections. J. Comp. Neurol. 422, 66–94 (2000)
Rorth, P. A modular misexpression screen in Drosophila detecting tissue-specific phenotypes. Proc. Natl Acad. Sci. USA 93, 12418–12422 (1996)
Helfrich-Forster, C. The period clock gene is expressed in central nervous system neurons which also produce a neuropeptide that reveals the projections of circadian pacemaker cells within the brain of Drosophila melanogaster. Proc. Natl Acad. Sci. USA 92, 612–616 (1995)
Veleri, S., Brandes, C., Helfrich-Forster, C., Hall, J. C. & Stanewsky, R. A self-sustaining, light-entrainable circadian oscillator in the Drosophila brain. Curr. Biol. 13, 1758–1767 (2003)
Yang, Z. & Sehgal, A. Role of molecular oscillations in generating behavioural rhythms in Drosophila. Neuron 29, 453–467 (2001)
Aton, S. J., Colwell, C. S., Harmar, A. J., Waschek, J. & Herzog, E. D. Vasoactive intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons. Nature Neurosci. 8, 476–483 (2005)
Petri, B. & Stengl, M. Pigment-dispersing hormone shifts the phase of the circadian pacemaker of the cockroach Leucophaea maderae. J. Neurosci. 17, 4087–4093 (1997)
Klarsfeld, A. et al. Novel features of cryptochrome-mediated photoreception in the brain circadian clock of Drosophila. J. Neurosci. 24, 1468–1477 (2004)
de la Iglesia, H. O., Cambras, T., Schwartz, W. J. & Diez-Noguera, A. Forced desynchronization of dual circadian oscillators within the rat suprachiasmatic nucleus. Curr. Biol. 14, 796–800 (2004)
Jagota, A., de la Iglesia, H. O. & Schwartz, W. J. Morning and evening circadian oscillations in the suprachiasmatic nucleus in vitro. Nature Neurosci. 3, 372–376 (2000)
Levine, J., Funes, P., Dowse, H. & Hall, J. Signal analysis of behavioural and molecular cycles. BMC Neurosci. 3, 1 (2002)
Levine, J., Funes, P., Dowse, H. & Hall, J. Advanced analysis of a cryptochrome mutation's effects on the robustness and phase of molecular cycles in isolated peripheral tissues of Drosophila. BMC Neurosci. 3, 5 (2002)
Zhao, J. et al. Drosophila clock can generate ectopic circadian clocks. Cell 113, 755–766 (2003)
Acknowledgements
We thank J. Hall, R. Allada, P. Emery and R. Stanewsky for critical comments on the manuscript; J. Agosto, J. Menet, E. Nagoshi and S. Kadener for discussions and advice; E. Dougherty for assistance in confocal microscopy; and H. Felton for administrative assistance. The work was supported in part by grants from the NIH to M.R.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
Supplementary information
Supplementary Figure 1
Analysis of DD locomotor rhythms of flies expressing SGG in different clock-cell groups. (PDF 355 kb)
Supplementary Figure 2
Temporal variation of tim RNA expression level in each clock neuronal group during the fourth day of constant darkness (DD4) in brains of EP1576 flies. (PDF 116 kb)
Supplementary Figure 3
Representative examples of whole-mount brain in-situ hybridization results in EP1576 flies. (PDF 254 kb)
Supplementary Figure 4
Temporal variation of tim RNA expression level during DD4 in each clock neuronal group in timGAL4/UAS-SGG brains. (PDF 109 kb)
Supplementary Figure 5
Representative whole-mount brain in-situ hybridization pictures of timGAL4/UAS-SGG flies. (PDF 222 kb)
Supplementary Figure 6
Temporal variation of tim RNA expression level in each clock neuronal group in PdfGAL4/UAS-SGG brains. (PDF 123 kb)
Supplementary Figure 7
Representative whole-mount brain in-situ hybridization pictures of PdfGAL4/ EP1576 flies. (PDF 299 kb)
Supplementary Figure 8
Temporal variation of tim RNA expression level in each clock neuronal group in timGAL4/UAS-SGG/PdfGAL80 brains. (PDF 126 kb)
Supplementary Figure 9
Representative whole-mount brain in-situ hybridization pictures of timGAL4/ EP1576/PdfGAL80 flies. (PDF 433 kb)
Supplementary Figure 10
Temporal variation of tim RNA expression level of each clock neuronal group in timGAL4/UAS-SGG/cryGAL80 brains. (PDF 116 kb)
Supplementary Figure 11
Representative whole-mount brain in-situ hybridization pictures of timGAL4/ EP1576/cryGAL80 flies. (PDF 367 kb)
Supplementary Figure Legends
Legends to accompany the above Supplementary Figures. (DOC 29 kb)
Rights and permissions
About this article
Cite this article
Stoleru, D., Peng, Y., Nawathean, P. et al. A resetting signal between Drosophila pacemakers synchronizes morning and evening activity. Nature 438, 238–242 (2005). https://doi.org/10.1038/nature04192
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature04192
This article is cited by
-
A four-oscillator model of seasonally adapted morning and evening activities in Drosophila melanogaster
Journal of Comparative Physiology A (2023)
-
Circuit analysis reveals a neural pathway for light avoidance in Drosophila larvae
Nature Communications (2022)
-
Integrated omics in Drosophila uncover a circadian kinome
Nature Communications (2020)
-
The optic lobe–pars intercerebralis axis is involved in circa’bi’dian rhythm of the large black chafer Holotrichia parallela
Journal of Comparative Physiology A (2020)
-
Nuclear Envelope Protein MAN1 Regulates the Drosophila Circadian Clock via Period
Neuroscience Bulletin (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.