Abstract
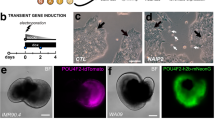
Lens regeneration in adult newts is a classic example of how cells can faithfully regenerate a complete organ through the process of transdifferentiation1,2,3,4,5,6. After lens removal, the pigment epithelial cells of the dorsal, but not the ventral, iris dedifferentiate and then differentiate to form a new lens. Understanding how this process is regulated might provide clues about why lens regeneration does not occur in higher vertebrates. The genes six-3 and pax-6 are known to induce ectopic lenses during embryogenesis7,8. Here we tested these genes, as well as members of the bone morphogenetic protein (BMP) pathway that regulate establishment of the dorsal–ventral axis in embryos9, for their ability to induce lens regeneration. We show that the lens can be regenerated from the ventral iris when the BMP pathway is inhibited and when the iris is transfected with six-3 and treated with retinoic acid. In intact irises, six-3 is expressed at higher levels in the ventral than in the dorsal iris. During regeneration, however, only expression in the dorsal iris is significantly increased. Such an increase is seen in ventral irises only when they are induced to transdifferentiate by six-3 and retinoic acid or by BMP inhibitors. These data suggest that lens regeneration can be achieved in noncompetent adult tissues and that this regeneration occurs through a gene regulatory mechanism that is more complex than the dorsal expression of lens regeneration-specific genes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Colucci, V. L. Sulla rigenerazione parziale dell'occhio nei Tritoni-Istogenesi e sviluppo. Studio sperimentale. Mem. R. Acad. Sci. 1st Bologna Ser. 51, 593–629 (1891)
Wolff, G. Entwicklungsphysiologische Studien. I. Die regeneration der urodelenlinse. Wilhelm Roux Arch. Entwickl-Mech. Org. 1, 380–390 (1895)
Tsonis, P. A. Regeneration in vertebrates. Dev. Biol. 221, 273–284 (2000)
Eguchi, G. Electron microscopic studies on lens regeneration I. Mechanism of depigmentation of the iris. Embryologia 8, 45–62 (1963)
Eguchi, G. Electron microscopic studies on lens regeneration. II. Formation and growth of lens vesicle and differentiation of lens fibers. Embryologia 8, 247–287 (1964)
Yamada, T. Control mechanisms in cell-type conversion in newt lens regeneration. Monogr. Dev. Biol. 13, 1–126 (1977)
Oliver, G., Loosli, F., Koster, R., Wittbrodt, J. & Gruss, P. Ectopic lens induction in fish in response to the murine homeobox gene six-3. Mech. Dev. 60, 233–239 (1996)
Altmann, C. R., Chow, R. L., Lang, R. A. & Hemmati-Brivanlou, A. Lens induction by Pax-6 in Xenopus laevis. Dev. Biol. 185, 119–123 (1997)
DeRobertis, E. M. & Kuroda, H. Dorsal–ventral patterning and neural induction in Xenopus embryos. Annu. Rev. Cell Dev. Biol. 20, 285–308 (2004)
Okamoto, M., Ito, M. & Owaribe, K. Difference between dorsal and ventral iris in lens producing potency in normal lens regeneration is maintained after dissociation and reaggregation of cells from the adult newt, Cynops pyrrhogaster. Develop. Growth Differ. 40, 11–18 (1998)
Ito, M., Hayashi, T., Kuroiwa, A. & Okamoto, M. Lens formation by pigmented epithelial cell reaggregate from dorsal iris implanted into limb blastema in the adult newt. Dev. Growth Differ. 41, 429–440 (1999)
Hayashi, T. et al. Highly efficient transfection system for functional gene analysis in adult amphibian lens regeneration. Develop. Growth Differ. 43, 361–370 (2001)
McCaffery, P., Lee, M., Wagner, M. A., Sladek, N. E. & Drager, U. C. Asymmetrical retinoic acid synthesis in the dorsoventral axis of the retina. Development 115, 371–382 (1992)
Tsonis, P. A. Limb Regeneration (Cambridge Univ. Press, Cambridge, 1996)
Tsonis, P. A., Trombley, M. T., Rowland, T., Chandraratna, R. A. S. & Del Rio-Tsonis, K. Role of retinoic acid in lens regeneration. Dev. Dyn. 219, 588–593 (2000)
Maden, M. & Hind, M. Retinoic acid, a regeneration-inducing molecule. Dev. Dyn. 226, 237–244 (2003)
Lee, S. H., Fu, K. K., Hui, J. N. & Richman, J. M. Noggin and retinoic acid transform the identity of avian facial prominences. Nature 414, 909–912 (2001)
Skillington, J., Choy, L. & Derynck, R. Bone morphogenetic protein and retinoic acid signalling cooperate to induce osteoblast differentiation of pre-adipocytes. J. Cell Biol. 159, 135–146 (2002)
Zuber, M. E. et al. Specification of the vertebrate eye by a network of eye field transcription factors. Development 130, 5155–5167 (2003)
Hayashi, T. et al. FGF2 triggers iris-derived lens regeneration in newt eye. Mech. Dev. 121, 519–526 (2004)
Tsonis, P. A. et al. A novel role of the hedgehog pathway in lens regeneration. Dev. Biol. 267, 450–451 (2004)
Pfaffl, M. W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 29, e45 (2001)
Sawada, K., Agata, K., Yoshiki, A. & Eguchi, G. A set of anti-crystallin monoclonal antibodies for detecting lens specificities: β-crystallin as a specific marker for detecting lentoidogenesis in culture of chicken lens epithelial cells. Japn. J. Ophthalmol. 37, 355–368 (1993)
Acknowledgements
We thank T. Hayashi for sharing his expertise with the culturing methods for PECs; G. Oliver and D. Englecamp for the six-3 and pax-6 expression vectors, respectively; members of our laboratories, M. Metheney, A. Thitoff, S. Gainer, M. Tsavaris, M. Tubb and M. Sander for help with histology; V. Mitashov and E. Makarev for providing information on P.Waltl six-3 sequences; and E. Tanaka for information on axolotl BMPR-IA sequences. This work was supported by a grant from the NIH (to P.A.T.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Sequences for six-3 and BMPR-IA have been deposited in GenBank under accession numbers AY799802 and AY795966, respectively. Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
Supplementary information
Supplementary Table 1
Describes the cases of induction of lens regeneration in PEC aggregates or explants in response to six-3 transfections and BMP treatments. (DOC 34 kb)
Supplementary Figure 1
Shows the efficiency of transfection and the expression of exogenous genes. (PDF 1448 kb)
Supplementary Figure Legend
Text to accompany the above Supplementary Figure. (DOC 24 kb)
Supplementary Methods
Several methods on the isolation, culture, treatment and transfection of PECs. (DOC 30 kb)
Rights and permissions
About this article
Cite this article
Grogg, M., Call, M., Okamoto, M. et al. BMP inhibition-driven regulation of six-3 underlies induction of newt lens regeneration. Nature 438, 858–862 (2005). https://doi.org/10.1038/nature04175
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature04175
This article is cited by
-
ITRAQ-based quantitative proteomic analysis of Cynops orientalis limb regeneration
BMC Genomics (2017)
-
Turning terminally differentiated skeletal muscle cells into regenerative progenitors
Nature Communications (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.