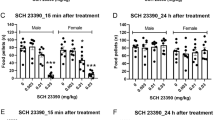
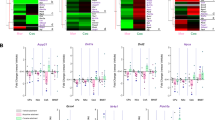
Abstract
Dopamine has been widely implicated as a mediator of many of the behavioural responses to drugs of abuse1. To test the hypothesis that dopamine is an essential mediator of various opiate-induced responses, we administered morphine to mice unable to synthesize dopamine. We found that dopamine-deficient mice are unable to mount a normal locomotor response to morphine, but a small dopamine-independent increase in locomotion remains. Dopamine-deficient mice have a rightward shift in the dose–response curve to morphine on the tail-flick test (a pain sensitivity assay), suggesting either a decreased sensitivity to the analgesic effects of morphine and/or basal hyperalgesia. In contrast, dopamine-deficient mice display a robust conditioned place preference for morphine when given either caffeine or l-dihydroxyphenylalanine (a dopamine precursor that restores dopamine throughout the brain) during the testing phases. Together, these data demonstrate that dopamine is a crucial component of morphine-induced locomotion, dopamine may contribute to morphine analgesia, but that dopamine is not required for morphine-induced reward as measured by conditioned place preference.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Spanagel, R. & Weiss, F. The dopamine hypothesis of reward: past and current status. Trends Neurosci. 22, 521–527 (1999)
Wise, R. A. Dopamine, learning and motivation. Nature Rev. Neurosci. 5, 483–494 (2004)
Berridge, K. C. & Robinson, T. E. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res. Brain Res. Rev. 28, 309–369 (1998)
Gysling, K. & Wang, R. Y. Morphine-induced activation of A10 dopamine neurons in the rat. Brain Res. 277, 119–127 (1983)
Di Chiara, G. & Imperato, A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc. Natl Acad. Sci. USA 85, 5274–5278 (1988)
Shippenberg, T. S., Bals-Kubik, R. & Herz, A. Examination of the neurochemical substrates mediating the motivational effects of opioids: role of the mesolimbic dopamine system and D-1 vs. D-2 dopamine receptors. J. Pharmacol. Exp. Ther. 265, 53–59 (1993)
Manzanedo, C., Aguilar, M. A., Rodriguez-Arias, M. & Minarro, J. Effects of dopamine antagonists with different receptor blockade profiles on morphine-induced place preference in male mice. Behav. Brain Res. 121, 189–197 (2001)
Pettit, H. O., Ettenberg, A., Bloom, F. E. & Koob, G. F. Destruction of dopamine in the nucleus accumbens selectively attenuates cocaine but not heroin self-administration in rats. Psychopharmacology (Berl.) 84, 167–173 (1984)
Bechara, A., Nader, K. & van der Kooy, D. A two-separate-motivational-systems hypothesis of opioid addiction. Pharmacol. Biochem. Behav. 59, 1–17 (1998)
Johnson, S. W. & North, R. A. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J. Neurosci. 12, 483–488 (1992)
Bozarth, M. A. Neuroanatomical boundaries of the reward-relevant opiate-receptor field in the ventral tegmental area as mapped by the conditioned place preference method in rats. Brain Res. 414, 77–84 (1987)
Bozarth, M. A. & Wise, R. A. Intracranial self-administration of morphine into the ventral tegmental area in rats. Life Sci. 28, 551–555 (1981)
Maldonado, R. et al. Absence of opiate rewarding effects in mice lacking dopamine D2 receptors. Nature 388, 586–589 (1997)
Elmer, G. I. et al. Failure of intravenous morphine to serve as an effective instrumental reinforcer in dopamine D2 receptor knock-out mice. J. Neurosci. 22, RC224 (2002)
Dockstader, C. L., Rubinstein, M., Grandy, D. K., Low, M. J. & van der Kooy, D. The D2 receptor is critical in mediating opiate motivation only in opiate-dependent and withdrawn mice. Eur. J. Neurosci. 13, 995–1001 (2001)
Zhou, Q. Y. & Palmiter, R. D. Dopamine-deficient mice are severely hypoactive, adipsic, and aphagic. Cell 83, 1197–1209 (1995)
Szczypka, M. S. et al. Feeding behaviour in dopamine-deficient mice. Proc. Natl Acad. Sci. USA 96, 12138–12143 (1999)
Murphy, N. P., Lam, H. A. & Maidment, N. T. A comparison of morphine-induced locomotor activity and mesolimbic dopamine release in C57BL6, 129Sv and DBA2 mice. J. Neurochem. 79, 626–635 (2001)
Heusner, C. L. et al. Viral restoration of dopamine to the nucleus accumbens is sufficient to induce a locomotor response to amphetamine. Brain Res. 980, 266–274 (2003)
Kim, D. S. & Palmiter, R. D. Adenosine receptor blockade reverses hypophagia and enhances locomotor activity of dopamine-deficient mice. Proc. Natl Acad. Sci. USA 100, 1346–1351 (2003)
Chudler, E. H. & Dong, W. K. The role of the basal ganglia in nociception and pain. Pain 60, 3–38 (1995)
Franklin, K. B. Analgesia and abuse potential: an accidental association or a common substrate? Pharmacol. Biochem. Behav. 59, 993–1002 (1998)
Altier, N. & Stewart, J. The role of dopamine in the nucleus accumbens in analgesia. Life Sci. 65, 2269–2287 (1999)
King, M. A., Bradshaw, S., Chang, A. H., Pintar, J. E. & Pasternak, G. W. Potentiation of opioid analgesia in dopamine2 receptor knock-out mice: evidence for a tonically active anti-opioid system. J. Neurosci. 21, 7788–7792 (2001)
Cannon, C. M. & Palmiter, R. D. Reward without dopamine. J. Neurosci. 23, 10827–10831 (2003)
Robinson, S., Sandstrom, S. M., Denenberg, V. H. & Palmiter, R. D. Distinguishing whether dopamine regulates liking, wanting, and/or learning about rewards. Behav. Neurosci. 119, 5–15 (2005)
Bechara, A. & van der Kooy, D. The tegmental pedunculopontine nucleus: a brain-stem output of the limbic system critical for the conditioned place preferences produced by morphine and amphetamine. J. Neurosci. 9, 3400–3409 (1989)
Olds, M. E. Reinforcing effects of morphine in the nucleus accumbens. Brain Res. 237, 429–440 (1982)
Fenu, S., Bassareo, V. & Di Chiara, G. A role for dopamine D1 receptors of the nucleus accumbens shell in conditioned taste aversion learning. J. Neurosci. 21, 6897–6904 (2001)
Acknowledgements
We thank our colleagues F. Perez for help generating mice, and E. Kremer for providing us with virus used for Supplementary Fig. 2. We thank the University of Washington NIDA center program for use of Noldus software. We thank our colleagues C. Chavkin, W. Watt and S. Luquet for comments on the manuscript. T.S.H. was supported in part by a grant from NIGMS.Author Contributions The mouse model was developed in the R.D.P. laboratory. These experiments were designed and executed by T.S.H. with input from R.D.P. and assistance from B.N.S. for experiments shown in Fig. 1b and Supplementary Fig. 1.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
Supplementary information
Supplementary Figure 1
Morphine (25 mg/kg) locomotor responses after caffeine (15 mg/kg) pre-treatment. (PDF 21 kb)
Supplementary Figure 2
CPP for morphine in vrDD and control mice. (PDF 15 kb)
Supplementary Figure Legends
Text to accompany the above Supplementary Figures. (DOC 21 kb)
Rights and permissions
About this article
Cite this article
Hnasko, T., Sotak, B. & Palmiter, R. Morphine reward in dopamine-deficient mice. Nature 438, 854–857 (2005). https://doi.org/10.1038/nature04172
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature04172
This article is cited by
-
Reduced dopamine release in Dcc haploinsufficiency male mice abolishes the rewarding effects of cocaine but not those of morphine and ethanol
Psychopharmacology (2023)
-
Unique pharmacodynamic properties and low abuse liability of the µ-opioid receptor ligand (S)-methadone
Molecular Psychiatry (2023)
-
Risk for opioid misuse in chronic pain patients is associated with endogenous opioid system dysregulation
Translational Psychiatry (2022)
-
Influence of G protein-biased agonists of μ-opioid receptor on addiction-related behaviors
Pharmacological Reports (2021)
-
Dopamine receptors in the anterior cingulate cortex implicate in nicotine enhanced morphine analgesia
Psychopharmacology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.