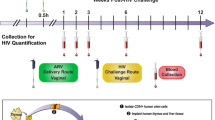
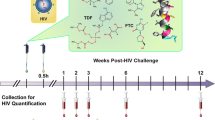
Abstract
Human immunodeficiency virus type 1 (HIV-1) continues to spread, principally by heterosexual sex, but no vaccine is available1. Hence, alternative prevention methods are needed to supplement educational and behavioural-modification programmes. One such approach is a vaginal microbicide: the application of inhibitory compounds before intercourse2. Here, we have evaluated the microbicide concept using the rhesus macaque ‘high dose’ vaginal transmission model with a CCR5-receptor-using simian–human immunodeficiency virus (SHIV-162P3) and three compounds that inhibit different stages of the virus–cell attachment and entry process. These compounds are BMS-378806, a small molecule that binds the viral gp120 glycoprotein and prevents its attachment to the CD4 and CCR5 receptors3,4, CMPD167, a small molecule that binds to CCR5 to inhibit gp120 association5, and C52L, a bacterially expressed peptide inhibitor of gp41-mediated fusion6. In vitro, all three compounds inhibit infection of T cells and cervical tissue explants, and C52L acts synergistically with CMPD167 or BMS-378806 to inhibit infection of cell lines. In vivo, significant protection was achieved using each compound alone and in combinations. CMPD167 and BMS-378806 were protective even when applied 6 h before challenge.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Klausner, R. D. et al. Medicine. The need for a global HIV vaccine enterprise. Science 300, 2036–2069 (2003)
Shattock, R. A. & Moore, J. P. Inhibiting HIV-1 sexual transmission. Nature Rev. Microbiol. 1, 25–34 (2003)
Lin, P. F. et al. A small molecule HIV-1 inhibitor that targets the HIV-1 envelope and inhibits CD4 receptor binding. Proc. Natl Acad. Sci. USA 100, 11013–11018 (2003)
Guo, Q. et al. Biochemical and genetic characterizations of a novel human immunodeficiency virus type 1 inhibitor that blocks gp120–CD4 interactions. J. Virol. 77, 10528–10536 (2003)
Shen, D. M. et al. Antagonists of human CCR5 receptor containing 4-(pyrazolyl)piperidine side chains. Part 2: Discovery of potent, selective, and orally bioavailable compounds. Bioorg. Med. Chem. Lett. 14, 941–945 (2004)
Lu, M. Stabilizing peptides and their use in the preparation of stabilized HIV inhibitors. World Intellectual Property Organization Patent WO-04/106364A1 (2004).
Seibert, C. & Sakmar, T. P. Small-molecule antagonists of CCR5 and CXCR4: A promising new class of anti-HIV-1 drugs. Curr. Pharmacol. Des. 10, 2041–2062 (2004)
Veazey, R. S. et al. Use of a small-molecule CCR5 inhibitor in macaques to treat simian immunodeficiency virus infection and prevent simian-human immunodeficiency virus infection. J. Exp. Med. 198, 1551–1562 (2003)
Wolinsky, S. M. et al. Effect of a CCR5 inhibitor on viral loads in macaques dual-infected with R5 and X4 primate immunodeficiency viruses. Virology 328, 19–29 (2004)
Hanna, G. et al. Antiviral activity, safety, and tolerability of a novel, oral small-molecule HIV-1 attachment inhibitor, BMS-488043, in HIV-1-infected subjects. Abstr. 141, 11th Conf. Retroviruses Opportunistic Infect. http://www.retroconference.org/Search_Abstract_2004/AbstractSearch.aspx (2004).
Matthews, T. et al. Enfuvirtide: The first therapy to inhibit the entry of HIV-1 into host CD4 lymphocytes. Nature Rev. Drug Disc. 3, 215–221 (2004)
Harouse, J. M. et al. Mucosal transmission and induction of simian AIDS by CCR5-specific simian/human immunodeficiency virus SHIVSF162P3 . J. Virol. 75, 1990–1995 (2001)
Moore, J. P., Kitchen, S. G., Pugach, P. & Zack, J. A. The CCR5 and CXCR4 coreceptors—central to understanding the transmission and pathogenesis of human immunodeficiency virus type 1 infection. AIDS Res. Hum. Retroviruses 20, 111–126 (2004)
Veazey, R. S. et al. Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV-1 gp120. Nature Med. 9, 343–346 (2003)
Lederman, M. M. et al. Prevention of vaginal SHIV transmission in rhesus macaques through inhibition of CCR5. Science 306, 485–487 (2004)
Tsai, C. C. et al. Cyanovirin-N inhibits AIDS virus infections in vaginal transmission models. AIDS Res. Hum. Retroviruses 20, 11–18 (2004)
Hu, Q. et al. Blockade of attachment and fusion receptors inhibits HIV-1 infection of human cervical tissue. J. Exp. Med. 199, 1065–1075 (2004)
Tremblay, C. Effects of HIV-1 entry inhibitors in combination. Curr. Pharm. Des. 10, 1861–1865 (2004)
Watson, C., Jenkinson, S., Kazmierski, W. & Kenakin, T. P. The CCR5 receptor-based mechanism of action of 873140, a potent allosteric non-competitive HIV entry-inhibitor. Mol. Pharmacol. 67, 1268–1282 (2005)
Sparks, S. et al. Prolonged duration of CCR5 occupancy by 873140 in HIV-negative and HIV-positive subjects. Abstr. 77, 12th Conf. Retroviruses Opportunistic Infect. http://www.retroconference.org/Search_Abstract_2005/AbstractSearch.aspx (2005).
Marx, P. A. et al. Progesterone implants enhance SIV vaginal transmission and early virus load. Nature Med. 2, 1084–1089 (1996)
Otten, R. A. et al. Multiple vaginal exposures to low doses of R5 simian-human immunodeficiency virus: strategy to study HIV preclinical interventions in nonhuman primates. J. Infect. Dis. 191, 164–173 (2005)
Dyer, J. R. et al. High levels of human immunodeficiency virus type 1 in blood and semen of seropositive men in sub-Saharan Africa. J. Infect. Dis. 177, 1742–1746 (1998)
Wawer, M. J. et al. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J. Infect. Dis. 191, 1403–1409 (2005)
Reeves, J. D. et al. Sensitivity of HIV-1 to entry inhibitors correlates with envelope/coreceptor affinity, receptor density, and fusion kinetics. Proc. Natl Acad. Sci. USA 99, 16249–16254 (2002)
Miller, C. J. et al. Propagation and dissemination of infection after vaginal transmission of simian immunodeficiency virus. J. Virol. 79, 9217–9227 (2005)
Pope, M. & Haase, A. T. Transmission, acute HIV-1 infection and the quest for strategies to prevent infection. Nature Med. 9, 847–852 (2003)
Ketas, T. J. et al. Entry inhibitors SCH-C, RANTES, and T-20 block HIV type 1 replication in multiple cell types. AIDS Res. Hum. Retroviruses 19, 177–186 (2003)
Wang, T. et al. Discovery of 4-Benzoyl-1-[(4-methoxy-1H-pyrrolo[2,3-b]pyridin-3-yl)oxoacetyl]-2-(R)-methylpiperazine (BMS-378806): A novel HIV-1 attachment inhibitor that interferes with CD4-gp120 interactions. J. Med. Chem. 46, 4236–4239 (2003)
Ji, H., Bracken, C. & Lu, M. Buried polar interactions and conformational stability in the simian immunodeficiency virus (SIV) gp41 core. Biochemistry 39, 676–685 (2000)
Acknowledgements
We thank J. LeBlanc, M. Dodd and T. Williams for technical support, and we appreciate receiving advice from M. Pope. This work was supported by an NIH grant, and by an Unrestricted Infectious Diseases Award from the Bristol-Myers Squibb Foundation to J.P.M. Author Contributions R.S.V. was responsible for designing, organizing and executing the macaque studies, which were carried out by J.D. P.A.M. provided advice on the macaque model and supervised the performance of serology studies on samples from the test monkeys. P.J.K. contributed to the design of the monkey and cell culture experiments and performed all the mathematical and statistical analyses. S.M.S. was responsible for in vitro studies of inhibitor combinations, and T.J.K. for infection-inhibition experiments with human and macaque PBMCs. Q.H. carried out the experiments involving cervical tissue explants and macrophages (including combination studies), under the supervision of R.J.S., who was also involved in the design and interpretation of the macaque studies. M.L. synthesized the C52L peptide and advised on its use. R.J.C. provided BMS-378806 and advised on its use. M.S.S. provided CMPD167 and advised on its use. J.P.M. made a lot of phone calls and sent out many emails.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
R.A.C. and M.S.S. are employees of Bristol-Myers Squibb Inc and Merck Inc, respectively, but neither company funded this study. M.L. has filed patents related to the C52L peptide.
Supplementary information
Supplementary Notes*
This section contains details on synergy between entry inhibitors in vitro, vaginal challenges with SHIV-162P4 and plasma viral load in SHIV-162P3-infected animals. * This Supplementary Information was substituted for an earlier version on 14/11/05. The replacement is identical, except that it contains a detailed directive for reagent requests on page 4. (PDF 253 kb)
Supplementary Figure 1
Challenge studies with SHIV-162P4, partial protection by CMPD167 and C52L. (PDF 385 kb)
Supplementary Figure 2
Plasma VL in SHIV-162P3-infected animals. (PDF 506 kb)
Rights and permissions
About this article
Cite this article
Veazey, R., Klasse, P., Schader, S. et al. Protection of macaques from vaginal SHIV challenge by vaginally delivered inhibitors of virus–cell fusion. Nature 438, 99–102 (2005). https://doi.org/10.1038/nature04055
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature04055
This article is cited by
-
Leukocytospermia induces intraepithelial recruitment of dendritic cells and increases SIV replication in colorectal tissue explants
Communications Biology (2021)
-
Innovation in the discovery of the HIV-1 attachment inhibitor temsavir and its phosphonooxymethyl prodrug fostemsavir
Medicinal Chemistry Research (2021)
-
High-level expression of the HIV entry inhibitor griffithsin from the plastid genome and retention of biological activity in dried tobacco leaves
Plant Molecular Biology (2018)
-
A novel preventive strategy against HIV-1 infection: combinatorial use of inhibitors targeting the nucleocapsid and fusion proteins
Emerging Microbes & Infections (2017)
-
CD4-mimetic sulfopeptide conjugates display sub-nanomolar anti-HIV-1 activity and protect macaques against a SHIV162P3 vaginal challenge
Scientific Reports (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.