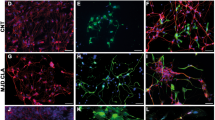
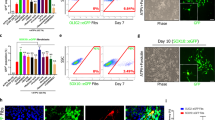
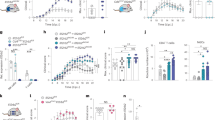
Abstract
In degenerative disorders of the central nervous system (CNS), transplantation of neural multipotent (stem) precursor cells (NPCs) is aimed at replacing damaged neural cells1,2. Here we show that in CNS inflammation, NPCs are able to promote neuroprotection by maintaining undifferentiated features and exerting unexpected immune-like functions. In a mouse model of chronic CNS inflammation, systemically injected adult syngeneic NPCs use constitutively activated integrins and functional chemokine receptors to selectively enter the inflamed CNS. These undifferentiated cells survive repeated episodes of CNS inflammation by accumulating within perivascular areas where reactive astrocytes, inflamed endothelial cells and encephalitogenic T cells produce neurogenic and gliogenic regulators. In perivascular CNS areas, surviving adult NPCs induce apoptosis of blood-borne CNS-infiltrating encephalitogenic T cells, thus protecting against chronic neural tissue loss as well as disease-related disability. These results indicate that undifferentiated adult NPCs have relevant therapeutic potential in chronic inflammatory CNS disorders because they display immune-like functions that promote long-lasting neuroprotection.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lindvall, O., Kokaia, Z. & Martinez-Serrano, A. Stem cell therapy for human neurodegenerative disorders-how to make it work. Nature Med. 10 (suppl.), S42–S50 (2004)
Pluchino, S. et al. Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature 422, 688–694 (2003)
McRae, B. L. et al. Induction of active and adoptive relapsing experimental autoimmune encephalomyelitis (EAE) using an encephalitogenic epitope of proteolipid protein. J. Neuroimmunol. 38, 229–240 (1992)
Parras, C. M. et al. Mash1 specifies neurons and oligodendrocytes in the postnatal brain. EMBO J. 23, 4495–4505 (2004)
Fukuda, S. et al. Two distinct subpopulations of nestin-positive cells in adult mouse dentate gyrus. J. Neurosci. 23, 9357–9366 (2003)
Lois, C., Garcia-Verdugo, J. M. & Alvarez-Buylla, A. Chain migration of neuronal precursors. Science 271, 978–981 (1996)
Wichterle, H., Garcia-Verdugo, J. M. & Alvarez-Buylla, A. Direct evidence for homotypic, glia-independent neuronal migration. Neuron 18, 779–791 (1997)
Alvarez-Buylla, A. & Lim, D. A. For the long run: maintaining germinal niches in the adult brain. Neuron 41, 683–686 (2004)
Jin, K. et al. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc. Natl Acad. Sci. USA 99, 11946–11950 (2002)
Constantin, G. et al. Chemokines trigger immediate β2 integrin affinity and mobility changes: differential regulation and roles in lymphocyte arrest under flow. Immunity 13, 759–769 (2000)
Constantin, G., Laudanna, C., Brocke, S. & Butcher, E. C. Inhibition of experimental autoimmune encephalomyelitis by a tyrosine kinase inhibitor. J. Immunol. 162, 1144–1149 (1999)
Alt, C., Laschinger, M. & Engelhardt, B. Functional expression of the lymphoid chemokines CCL19 (ELC) and CCL 21 (SLC) at the blood–brain barrier suggests their involvement in G-protein-dependent lymphocyte recruitment into the central nervous system during experimental autoimmune encephalomyelitis. Eur. J. Immunol. 32, 2133–2144 (2002)
Karpus, W. J. & Kennedy, K. J. MIP-1α and MCP-1 differentially regulate acute and relapsing autoimmune encephalomyelitis as well as Th1/Th2 lymphocyte differentiation. J. Leukoc. Biol. 62, 681–687 (1997)
Furlan, R. et al. Intrathecal delivery of IFN-γ protects C57BL/6 mice from chronic-progressive experimental autoimmune encephalomyelitis by increasing apoptosis of central nervous system-infiltrating lymphocytes. J. Immunol. 167, 1821–1829 (2001)
Weishaupt, A. et al. Molecular mechanisms of high-dose antigen therapy in experimental autoimmune encephalomyelitis: rapid induction of Th1-type cytokines and inducible nitric oxide synthase. J. Immunol. 165, 7157–7163 (2000)
Marsden, V. S. & Strasser, A. Control of apoptosis in the immune system: Bcl-2, BH3-only proteins and more. Annu. Rev. Immunol. 21, 71–105 (2003)
Ricci-Vitiani, L. et al. Absence of caspase 8 and high expression of PED protect primitive neural cells from cell death. J. Exp. Med. 200, 1257–1266 (2004)
Schere-Levy, C. et al. Leukemia inhibitory factor induces apoptosis of the mammary epithelial cells and participates in mouse mammary gland involution. Exp. Cell Res. 282, 35–47 (2003)
Tarrant, T. K. et al. Interleukin 12 protects from a T helper type 1-mediated autoimmune disease, experimental autoimmune uveitis, through a mechanism involving interferon gamma, nitric oxide, and apoptosis. J. Exp. Med. 189, 219–230 (1999)
Pender, M. P. & Rist, M. J. Apoptosis of inflammatory cells in immune control of the nervous system: role of glia. Glia 36, 137–144 (2001)
Chu, K., Kim, M., Jeong, S. W., Kim, S. U. & Yoon, B. W. Human neural stem cells can migrate, differentiate, and integrate after intravenous transplantation in adult rats with transient forebrain ischemia. Neurosci. Lett. 343, 129–133 (2003)
Akiyama, Y. et al. Transplantation of clonal neural precursor cells derived from adult human brain establishes functional peripheral myelin in the rat spinal cord. Exp. Neurol. 167, 27–39 (2001)
Vandenbark, A. A. et al. Differential susceptibility of human Th1 versus Th2 cells to induction of anergy and apoptosis by ECDI/antigen-coupled antigen-presenting cells. Int. Immunol. 12, 57–66 (2000)
Zhang, X. et al. Unequal death in T helper cell (Th)1 and Th2 effectors: Th1, but not Th2, effectors undergo rapid Fas/FasL-mediated apoptosis. J. Exp. Med. 185, 1837–1849 (1997)
Merkle, F. T., Tramontin, A. D., Garcia-Verdugo, J. M. & Alvarez-Buylla, A. Radial glia give rise to adult neural stem cells in the subventricular zone. Proc. Natl Acad. Sci. USA 101, 17528–17532 (2004)
Constantin, G., Laudanna, C. & Butcher, E. C. Novel method for following lymphocyte traffic in mice using [3H]glycerol labeling. J. Immunol. Methods 203, 35–44 (1997)
Lazarini, F. et al. Differential signalling of the chemokine receptor CXCR4 by stromal cell-derived factor 1 and the HIV glycoprotein in rat neurons and astrocytes. Eur. J. Neurosci. 12, 117–125 (2000)
Acknowledgements
We wish to thank R. Galli, A. Gritti, M. Muzio, A. Vescovi and L. Ricci-Vitiani for discussions. We are grateful to F. Mavilio for critically discussing the manuscript. We acknowledge the technical help of S. Bach, S. Bucello, E. Butti, C. Covino, R. Molteni, A. Palini and C. Sciorati. S.P. is the recipient of a fellowship from the National Multiple Sclerosis Society (NMSS). L.Z. is the recipient of a fellowship from The Myelin Project (TMP). This work was supported in part by the Italian Minister of Health, the Italian Multiple Sclerosis Foundation (FISM), NMSS and TMP.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
Supplementary information
Supplementary Figure S1
I.v.-injection of syngenic aNPCs reduces clinical relapses in R-EAE mice. (PDF 57 kb)
Supplementary Figure S2
I.v.-injected syngenic aNPCs persist within CNS perivascular areas of R-EAE mice. (PDF 13711 kb)
Supplementary Figure S3
Confocal images showing co-localization experiments performed in aNPC-transplanted R-EAE mice 106 dpi. (PDF 4000 kb)
Supplementary Figure S4
Perivascular CNS areas from R-EAE mice express stem cell regulators and contain activated microglia. (PDF 17263 kb)
Supplementary Figure S5
Stem cell regulators co-localize with i.v.-injected aNPCs persisting within perivascular CNS areas from R-EAE mice. (PDF 17596 kb)
Supplementary Figure S6
aNPCs constitutively express VLA-4 and adhere to VCAM-1 expressing CNS inflamed microvessels from C57Bl/6 mice with chronic progressive EAE. (PDF 555 kb)
Supplementary Figure S7
aNPCs express wide range of functional pro-inflammatory chemokine receptors. (PDF 81 kb)
Supplementary Figure S8
In vitro and in vivo analysis of CD3+ cells undergoing apoptosis. (PDF 11511 kb)
Supplementary Figure S9
Expression of regulators of stem cell proliferation and differentiation, immune molecules and trophic factors by aNPCs. (PDF 55 kb)
Supplementary Figures Legends
Full text legends to accompany the above Supplementary Figures. (DOC 39 kb)
Supplementary Video S1
Animated 3D reconstruction of a CNS perivascular area showing persistence of β-gal+ cells in close contact to endothelial cells producing BMP-4. (MOV 235 kb)
Supplementary Video S2
Animated 3D reconstruction of a CNS perivascular area showing persistence of β-gal+ cells in close contact with CNS-infiltrating CD45+ cells producing Noggin. (MOV 246 kb)
Supplementary Video S3
Animated 3D reconstruction of a β-gal+ cell expressing PSA-NCAM and incorporating BrdU 106 dpi. (MOV 96 kb)
Supplementary Video S4
Animated 3D reconstruction of a β-gal+ cell incorporating BrdU 106 dpi. (MOV 134 kb)
Supplementary Methods
Additional descriptions of methods used in this study. (DOC 63 kb)
Rights and permissions
About this article
Cite this article
Pluchino, S., Zanotti, L., Rossi, B. et al. Neurosphere-derived multipotent precursors promote neuroprotection by an immunomodulatory mechanism. Nature 436, 266–271 (2005). https://doi.org/10.1038/nature03889
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature03889
This article is cited by
-
Graft-derived neurons and bystander effects are maintained for six months after human iPSC-derived NESC transplantation in mice’s cerebella
Scientific Reports (2024)
-
Blood-spinal cord barrier disruption in degenerative cervical myelopathy
Fluids and Barriers of the CNS (2023)
-
Subventricular zone cytogenesis provides trophic support for neural repair in a mouse model of stroke
Nature Communications (2023)
-
The Effects of Intranasal Implantation of Mesenchymal Stem Cells on Nitric Monoxide Levels in the Hippocampus, Control of Cognitive Functions, and Motor Activity in a Model of Cerebral Ischemia in Rats
BioNanoScience (2023)
-
Cell replacement therapy with stem cells in multiple sclerosis, a systematic review
Human Cell (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.