Abstract
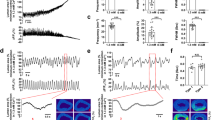
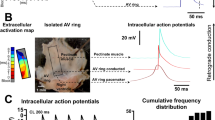
In excitable tissues the refractory period is a critical control mechanism preventing hyperactivity and undesirable tetani, by preventing subsequent stimuli eliciting action potentials and Ca2+ entry. In ureteric smooth muscle, peristaltic waves that occur as invading pacemaker potentials produce long-lasting action potentials (300–800 ms) and extraordinarily long (more than 10 s) refractory periods1,2,3,4,5,6, which prevent urine reflux and kidney damage2. For smooth muscles neither the mechanisms underlying the refractory period nor the link between excitability and refractoriness are properly understood. Here we show that a negative feedback process, which depends on Ca2+ loading the sarcoplasmic reticulum (SR) during the action potential and on the subsequent activation of local releases of Ca2+ from the SR (sparks7), stimulating plasmalemmal Ca2+-sensitive K+ (BK) channels, determines the refractory period of the action potential. As sparks gradually reduce the Ca2+ load in the SR, electrical inhibition is released, the refractory period is terminated and peristaltic contractions occur again. The refractory period can be manipulated, for example from 10 s to 100 s, by altering the Ca2+ content of the SR or release mechanism or by inhibiting BK channels. This insight into the control of excitability and hence function provides a focus for therapies directed at pathologies of smooth muscle.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Wemyss-Holden, G. D., Rose, M. R., Payne, S. R. & Testa, H. J. Non-invasive investigation of normal individual ureteric activity in man. Br. J. Urol. 71, 156–160 (1993)
Weiss, R. M. Campbell's Urology (ed. Walsh, P. C.) 94–128, (W.B. Saunders Company, Philadelphia, 1986)
Exintaris, B. & Lang, R. J. K. (+ ) channel blocker modulation of the refractory period in spontaneously active guinea-pig ureters. Urol. Res. 27, 319–327 (1999)
Kuriyama, H., Osa, T. & Toida, N. Membrane properties of the smooth muscle of guinea-pig ureter. J. Physiol. (Lond.) 191, 225–238 (1967)
Cuthbert, A. W. The relation between response and the interval between stimuli of the isolated guinea-pig ureter. J. Physiol. (Lond.) 180, 225–238 (1965)
Maggi, C. A., Giuliani, S. & Santicioli, P. Effect of Bay K 8644 and ryanodine on the refractory period, action potential and mechanical response of the guinea-pig ureter to electrical stimulation. Naunyn-Schmiedebergs Arch. Physiol. 349, 510–522 (1994)
Nelson, M. T. et al. Relaxation of arterial smooth muscle by calcium sparks. Science 270, 633–637 (1995)
Cheng, H., Lederer, W. J. & Cannell, M. B. Calcium sparks: elementary events underlying excitation–contraction coupling in heart muscle. Science 262, 740–744 (1993)
Burdyga, T. V., Taggart, M. J., Crichton, C., Smith, G. L. & Wray, S. The mechanism of Ca2+ release from the SR of permeabilised guinea-pig and rat ureteric smooth muscle. Biochim. Biophys. Acta 1402, 109–114 (1998)
Benham, C. D. & Bolton, T. B. Spontaneous transient outward currents in single visceral and vascualr smooth muscle cells of the rabbit. J. Physiol. (Lond.) 381, 385–406 (1986)
Imaizumi, Y., Muraki, K. & Watanabe, M. Ionic currents in single smooth muscle cells from the ureter of the guinea-pig. J. Physiol. (Lond.) 411, 131–159 (1989)
Lang, R. J. Identification of the major membrane currents in freshly dispersed single smooth muscle cells of guinea-pig ureter. J. Physiol. (Lond.) 412, 375–395 (1989)
Brenner, R. et al. Vasoregulation by the B1 subunit of the calcium-activated potassium channel. Nature 407, 870–876 (2000)
Wellman, G. C., Santana, L. F., Bonev, A. D. & Nelson, M. T. Role of the phospholamban in the modulation of arterial Ca2+ sparks and Ca2+-activated K+ channels by cAMP. Am. J. Physiol. Cell Physiol. 281, C1029–C1037 (2001)
Gordienko, D. V., Greenwood, I. A. & Bolton, T. B. Direct visualization of sarcoplasmic reticulum regions discharging Ca2+ sparks in vascular myocytes. Cell Calcium 29, 13–28 (2001)
Maggi, C. A., Giuliani, S. & Santicioli, P. Effect of the Ca2+-ATPase inhibitor, cyclopiazonic acid, on electromechanical coupling in the guinea-pig ureter. Br. J. Pharmacol. 114, 127–137 (1995)
Cheranov, S. Y. & Jaggar, J. H. Sarocplasmic reticulum calcium load regulates rat arterial smooth muscle calcium sparks and transient KCa currents. J. Physiol. (Lond.) 544, 71–84 (2002)
ZhuGe, R. et al. Ca2+ spark sites in smooth muscle cells are numerous and differ in number of ryanodine receptors, large-conductance K+ channels, and coupling ratio between them. Am. J. Physiol. Cell Physiol. 287, C1577–C1588 (2004)
Kotlikoff, M. I. Calcium-induced calcium release in smooth muscle: the case for loose coupling. Prog. Biophys. Mol. Biol. 83, 171–191 (2003)
Matthew, A. J. G., Kupittayanant, S., Burdyga, T. V. & Wray, S. Characterization of contractile activity and intracellular Ca2+ signalling in mouse myometrium. J. Soc. Gynecol. Investig. 11, 207–212 (2004)
Heppner, T. J., Bonev, A. D. & Nelson, M. T. Ca2+-activated K+ channels regulate action potential repolarization in urinary bladder smooth muscle. Am. J. Physiol. 273, C110–C117 (1997)
Bogdanov, K. Y., Vinogradova, T. M. & Lakatta, E. G. Sinoatrial nodal cell ryanodine receptor and Na+-Ca2+ exchanger: molecular partners in pacemaker regulation. Circ. Res. 88, 1254–1258 (2001)
Zima, A. V. & Blatter, L. A. Inositol-1,4,5-trisphosphate-dependent Ca2+ signalling in cat atrial excitation–contraction coupling and arrhythmias. J. Physiol. (Lond.) 555, 607–615 (2004)
Hove-Madsen, L. et al. Atrial fibrillation is associated with increased spontaneous calcium release from the sarcoplasmic reticulum in human atrial myocytes. Circulation 110, 1358–1363 (2004)
Shabir, S., Borisova, L., Wray, S. & Burdyga, T. Rho-kinase inhibition and electromechanical coupling in phasic smooth muscle; Ca2+-dependent and independent mechanisms. J. Physiol. (Lond.) 560, 839–855 (2004)
Burdyga, T. V. & Wray, S. Simultaneous measurements of electrical activity, intracellular [Ca2+] and force in intact smooth muscle. Pflügers Arch. 435, 182–184 (1997)
Acknowledgements
We thank D. Eisner and J. McCarron for discussions, and A. Shmygol and L. Borisova for technical help. Funding was provided by the MRC.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
Supplementary information
Supplementary Video S1
Pseudo-colour images of Ca2+ transients in intact, Fluo-4 loaded guinea- pig ureter, evoked every 10s by suprathreshold depolarising pulses. (MOV 2858 kb)
Supplementary Video S2
An example of real-time grey colour images of Ca2+ sparks, which are discharged at frequent discharging sites located mainly at the edge of the cell membrane of individual smooth muscle cells. (MOV 1340 kb)
Supplementary Video S1 Legend
Legend to accompany this video. (PPT 134 kb)
Supplementary Video S2 Legend
Legend to accompany this video. (PPT 102 kb)
Rights and permissions
About this article
Cite this article
Burdyga, T., Wray, S. Action potential refractory period in ureter smooth muscle is set by Ca sparks and BK channels. Nature 436, 559–562 (2005). https://doi.org/10.1038/nature03834
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature03834
This article is cited by
-
Ryanodine receptor 2 contributes to hemorrhagic shock-induced bi-phasic vascular reactivity in rats
Acta Pharmacologica Sinica (2014)
-
Role of Ca2+ entry and Ca2+ stores in atypical smooth muscle cell autorhythmicity in the mouse renal pelvis
British Journal of Pharmacology (2007)
-
Erratum: Action potential refractory period in ureter smooth muscle is set by Ca sparks and BK channels
Nature (2005)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.