Abstract
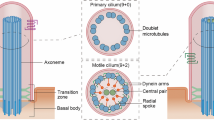
Cilia have diverse roles in motility and sensory reception, and defects in cilia function contribute to ciliary diseases such as Bardet–Biedl syndrome (BBS). Intraflagellar transport (IFT) motors assemble and maintain cilia by transporting ciliary precursors, bound to protein complexes called IFT particles, from the base of the cilium to their site of incorporation at the distal tip1,2,3. In Caenorhabditis elegans, this is accomplished by two IFT motors, kinesin-II and osmotic avoidance defective (OSM)-3 kinesin, which cooperate to form two sequential anterograde IFT pathways that build distinct parts of cilia4,5,6,7. By observing the movement of fluorescent IFT motors and IFT particles along the cilia of numerous ciliary mutants, we identified three genes whose protein products mediate the functional coordination of these motors. The BBS proteins BBS-7 and BBS-8 are required to stabilize complexes of IFT particles containing both of the IFT motors, because IFT particles in bbs-7 and bbs-8 mutants break down into two subcomplexes, IFT-A and IFT-B, which are moved separately by kinesin-II and OSM-3 kinesin, respectively. A conserved ciliary protein, DYF-1, is specifically required for OSM-3 kinesin to dock onto and move IFT particles, because OSM-3 kinesin is inactive and intact IFT particles are moved by kinesin-II alone in dyf-1 mutants. These findings implicate BBS ciliary disease proteins and an OSM-3 kinesin activator in the formation of two IFT pathways that build functional cilia.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Rosenbaum, J. L. & Witman, G. B. Intraflagellar transport. Nature Rev. Mol. Cell Biol. 3, 813–825 (2002)
Scholey, J. M. Intraflagellar transport. Annu. Rev. Cell Dev. Biol. 19, 423–443 (2003)
Pan, J., Wang, Q. & Snell, W. J. Cilium-generated signalling and Cilia-related disorders. Lab. Invest. 85, 452–463 (2005)
Cole, D. G. et al. Novel heterotrimeric kinesin-related protein purified from sea urchin eggs. Nature 366, 268–270 (1993)
Cole, D. G. et al. Chlamydomonas kinesin-II-dependent intraflagellar transport (IFT): IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons. J. Cell Biol. 141, 993–1008 (1998)
Signor, D., Wedaman, K. P., Rose, L. S. & Scholey, J. M. Two heteromeric kinesin complexes in chemosensory neurons and sensory cilia of Caenorhabditis elegans. Mol. Biol. Cell 10, 345–360 (1999)
Snow, J. J. et al. Two anterograde intraflagellar transport motors cooperate to build sensory cilia on C. elegans neurons. Nature Cell Biol. 6, 1109–1113 (2004)
Vale, R. D. The molecular motor toolbox for intracellular transport. Cell 112, 467–480 (2003)
Miki, H., Setou, M., Kaneshiro, K. & Hirokawa, N. All kinesin superfamily protein, KIF, genes in mouse and human. Proc. Natl Acad. Sci. USA 98, 7004–7011 (2001)
Goldstein, L. S. B. & Yang, Z. Microtubule-based transport systems in neurons. The roles of kinesins and dyneins. Annu. Rev. Cell Dev. Biol. 23, 39–71 (2000)
Mallik, R. & Gross, S. P. Molecular motors: strategies to get along. Curr. Biol. 14, R971–R982 (2004)
Lawrence, C. J. et al. A standardized kinesin nomenclature. J. Cell Biol. 167, 19–22 (2004)
Tabish, M., Siddiqui, Z. K., Nishikawa, K. & Siddiqui, S. Exclusive expression of C. elegans osm-3 kinesin gene in chemosensory neurons open to the external environment. J. Mol. Biol. 247, 377–389 (1995)
Perkins, L. A., Hedgecock, E. M., Thomson, J. N. & Culotti, J. G. Mutant sensory cilia in the nematode Caenorhabditis elegans. Dev. Biol. 117, 456–487 (1986)
Starich, T. A. et al. Mutations affecting chemosensory neurons of Caenorhabditis elegans. Genetics 139, 171–188 (1995)
Scholey, J. M., Ou, G., Snow, J. J. & Gunnarson, A. Intraflagellar transport motors in Caenorhabditis elegans neurons. Biochem. Soc. Trans. 32, 682–684 (2004)
Blacque, O. E. et al. Loss of C. elegans BBS-7 and BBS-8 protein function results in cilia defects and compromised intraflagellar transport. Genes Dev. 18, 1630–1642 (2004)
De Riso, L., Ristoratore, F., Sebastiano, M. & Bazzicalupo, P. Amphid defective mutant of Caenorhabditis elegans. Genetica 94, 195–202 (1994)
Swoboda, P., Adler, H. T. & Thomas, J. H. The RFX-type transcription factor DAF-19 regulates sensory neuron cilium formation in C. elegans. Mol. Cell 5, 411–421 (2000)
Li, J. B. et al. Comparative genomics identifies a flagellar and basal body proteome that includes the BBS5 human disease gene. Cell 117, 541–552 (2004)
Orozco, J. T. et al. Movement of motor and cargo along cilia. Nature 398, 674 (1999)
Signor, D. et al. Role of a class DHC1b dynein in retrograde transport of IFT motors and IFT raft particles along cilia, but not dendrites, in chemosensory neurons of living Caenorhabditis elegans. J. Cell Biol. 147, 519–530 (1999)
Mueller, J., Perrone, C. A., Bower, R., Cole, D. G. & Porter, M. E. The FLA3 KAP subunit is required for localization of kinesin-2 to the site of flagellar assembly and processive anterograde intraflagellar transport. Mol. Biol. Cell 16, 1341–1354 (2005)
Deacon, S. W. et al. Dynactin is required for bidirectional organelle transport. J. Cell Biol. 160, 297–301 (2003)
Awan, A., Bernstein, M., Hamasaki, T. & Satir, P. Cloning and characterization of Kin5, a novel Tetrahymena ciliary kinesin II. Cell. Motil. Cytoskel. 58, 1–9 (2004)
Cole, D. G. The intraflagellar transport machinery of Chlamydomonas reinhardtii. Traffic 4, 435–442 (2003)
Mesland, D. A. M., Hoffman, J. L., Caligor, E. & Goodenough, U. W. Flagellar tip activation stimulated by membrane adhesions in Chlamydomonas gametes. J. Cell Biol. 84, 599–617 (1980)
Hobert, O. PCR fusion-based approach to create reporter gene constructs for expression analysis in transgenic C. elegans. Biotechniques 32, 728–730 (2002)
Acknowledgements
We thank W. M. Saxton, G. Rogers, D. Sharp, S. Gross, L. S. Rose and many other colleagues for comments. We thank E. Schwarz and M. Barr for help with DYF-1 protein domain analysis and T. Stiernagle, A. Fire, Y. Kohara and A. Coulson for reagents. This work was supported by grants from the Michael Smith Foundation (O.E.B. and M.R.L.), Canadian Institutes of Health Research and Heart and Stroke Foundation of B.C. & Yukon (M.R.L.) and the National Institutes of Health (J.M.S.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
Supplementary information
Supplementary Figure S1
DYF-1 homologs are present in various ciliated organisms but not in non-ciliated ones. (PDF 117 kb)
Supplementary Figure S2
Dissociation of IFT-motors and IFT subcomplex A and B components in bbs-7 and bbs-8 mutants. (PDF 797 kb)
Supplementary Video S1
Transport of DYF-1 and BBS proteins (DYF-1::GFP) along sensory cilia of wild-type. The display rate is 10 frames per second with total elapsed time of 60s. (MOV 3350 kb)
Supplementary Video S2
Transport of DYF-1 and BBS proteins (BBS-7::GFP) along sensory cilia of wild-type. The display rate is 10 frames per second with total elapsed time of 60s. (MOV 3167 kb)
Supplementary Video S3
Transport of DYF-1 and BBS proteins (BBS-8::GFP) along sensory cilia of wild-type. The display rate is 10 frames per second with total elapsed time of 60s. (MOV 4217 kb)
Supplementary Videos S4
Motility of IFT motors Kinesin-II (KAP-1::GFP) along sensory cilia in bbs-7(n1606) mutant. The display rate is 10 frames per second with total elapsed time of 60s. (MOV 2784 kb)
Supplementary Videos S5
Motility of IFT motors OSM-3-kinesin (OSM-3::GFP) along sensory cilia in bbs-7(n1606) mutant. The display rate is 10 frames per second with total elapsed time of 60s. (MOV 2214 kb)
Supplementary Video S6
Transport of IFT-particle A subcomplex (CHE-11::GFP) along sensory cilia of WT. The display rate is 10 frames per second with total elapsed time of 60s. (MOV 4303 kb)
Supplementary Video S7
Transport of IFT-particle A subcomplex (CHE-11::GFP) along sensory cilia of bbs-7(n1606) mutant. The display rate is 10 frames per second with total elapsed time of 60s. (MOV 3037 kb)
Supplementary Video S8
Transport of IFT-particle B subcomplex (CHE-2::GFP) along sensory cilia of WT. The display rate is 10 frames per second with total elapsed time of 60s. (MOV 4021 kb)
Supplementary Video S9
Transport of IFT-particle B subcomplex (CHE-2::GFP) along sensory cilia of bbs-7(n1606) mutant. The display rate is 10 frames per second with total elapsed time of 60s. (MOV 3451 kb)
Supplementary Video S10
Motility of IFT-particle (OSM-6::GFP) along sensory cilia in dyf-1(mn335) mutant. The display rate is 10 frames per second with total elapsed time of 60s. (MOV 3694 kb)
Supplementary Video S11
Motility of IFT motors Kinesin-II (KAP-1::GFP) along sensory cilia in dyf-1(mn335) mutant. The display rate is 10 frames per second with total elapsed time of 60s. (MOV 3237 kb)
Supplementary Video S12
Motility of OSM-3-kinesin (OSM-3::GFP) along sensory cilia in dyf-1(mn335) mutant. The display rate is 10 frames per second with total elapsed time of 60s. (MOV 2857 kb)
Supplementary Video S13
Transport of DYF-1::GFP along sensory cilia of osm-3(p802) mutant. The display rate is 10 frames per second with total elapsed time of 60s. (MOV 3289 kb)
Supplementary Video S14
Transport of DYF-1::GFP along sensory cilia of klp-11(tm324) mutant. The display rate is 10 frames per second with total elapsed time of 60s. (MOV 4013 kb)
Rights and permissions
About this article
Cite this article
Ou, G., E. Blacque, O., Snow, J. et al. Functional coordination of intraflagellar transport motors. Nature 436, 583–587 (2005). https://doi.org/10.1038/nature03818
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature03818
This article is cited by
-
Cilia regeneration requires an RNA splicing factor from the ciliary base
Cell Regeneration (2022)
-
Thermosensation in Caenorhabditis elegans is linked to ubiquitin-dependent protein turnover via insulin and calcineurin signalling
Nature Communications (2022)
-
STORM imaging reveals the spatial arrangement of transition zone components and IFT particles at the ciliary base in Tetrahymena
Scientific Reports (2021)
-
Cellular signalling by primary cilia in development, organ function and disease
Nature Reviews Nephrology (2019)
-
ERICH3 in Primary Cilia Regulates Cilium Formation and the Localisations of Ciliary Transport and Sonic Hedgehog Signaling Proteins
Scientific Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.