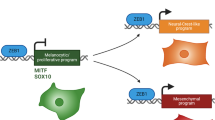
Abstract
Systematic analyses of cancer genomes promise to unveil patterns of genetic alterations linked to the genesis and spread of human cancers. High-density single-nucleotide polymorphism (SNP) arrays enable detailed and genome-wide identification of both loss-of-heterozygosity events and copy-number alterations in cancer1,2,3,4,5. Here, by integrating SNP array-based genetic maps with gene expression signatures derived from NCI60 cell lines, we identified the melanocyte master regulator MITF (microphthalmia-associated transcription factor) as the target of a novel melanoma amplification. We found that MITF amplification was more prevalent in metastatic disease and correlated with decreased overall patient survival. BRAF mutation and p16 inactivation accompanied MITF amplification in melanoma cell lines. Ectopic MITF expression in conjunction with the BRAF(V600E) mutant transformed primary human melanocytes, and thus MITF can function as a melanoma oncogene. Reduction of MITF activity sensitizes melanoma cells to chemotherapeutic agents. Targeting MITF in combination with BRAF or cyclin-dependent kinase inhibitors may offer a rational therapeutic avenue into melanoma, a highly chemotherapy-resistant neoplasm. Together, these data suggest that MITF represents a distinct class of ‘lineage survival’ or ‘lineage addiction’ oncogenes required for both tissue-specific cancer development and tumour progression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lindblad-Toh, K. et al. Loss-of-heterozygosity analysis of small-cell lung carcinomas using single-nucleotide polymorphism arrays. Nature Biotechnol. 18, 1001–1005 (2000)
Lieberfarb, M. E. et al. Genome-wide loss of heterozygosity analysis from laser capture microdissected prostate cancer using single nucleotide polymorphic allele (SNP) arrays and a novel bioinformatics platform dChipSNP. Cancer Res. 63, 4781–4785 (2003)
Mei, R. et al. Genome-wide detection of allelic imbalance using human SNPs and high-density DNA arrays. Genome Res. 10, 1126–1137 (2000)
Zhao, X. et al. An integrated view of copy number and allelic alterations in the cancer genome using single nucleotide polymorphism arrays. Cancer Res. 64, 3060–3071 (2004)
Bignell, G. R. et al. High-resolution analysis of DNA copy number using oligonucleotide microarrays. Genome Res. 14, 287–295 (2004)
Stinson, S. F. et al. Morphological and immunocytochemical characteristics of human tumour cell lines for use in a disease-oriented anticancer drug screen. Anticancer Res. 12, 1035–1053 (1992)
Ross, D. T. et al. Systematic variation in gene expression patterns in human cancer cell lines. Nature Genet. 24, 227–235 (2000)
Roschke, A. V. et al. Karyotypic complexity of the NCI-60 drug-screening panel. Cancer Res. 63, 8634–8647 (2003)
Nishizuka, S. et al. Proteomic profiling of the NCI-60 cancer cell lines using new high-density reverse-phase lysate microarrays. Proc. Natl Acad. Sci. USA 100, 14229–14234 (2003)
Monks, A., Scudiero, D. A., Johnson, G. S., Paull, K. D. & Sausville, E. A. The NCI anti-cancer drug screen: a smart screen to identify effectors of novel targets. Anticancer Drug Des. 12, 533–541 (1997)
Eisen, M. B., Spellman, P. T., Brown, P. O. & Botstein, D. Cluster analysis and display of genome-wide expression patterns. Proc. Natl Acad. Sci. USA 95, 14863–14868 (1998)
Golub, T. R. et al. Molecular classification of cancer: class discovery and class prediction by gene expression monitoring. Science 286, 531–537 (1999)
Steingrimsson, E., Copeland, N. G. & Jenkins, N. A. Melanocytes and the Microphthalmia Transcription Factor Network. Annu. Rev. Genet. 38, 365–411 (2004)
Camp, R. L., Dolled-Filhart, M., King, B. L. & Rimm, D. L. Quantitative analysis of breast cancer tissue microarrays shows that both high and normal levels of HER2 expression are associated with poor outcome. Cancer Res. 63, 1445–1448 (2003)
Hemesath, T. J., Price, E. R., Takemoto, C., Badalian, T. & Fisher, D. E. MAP kinase links the transcription factor Microphthalmia to c-Kit signalling in melanocytes. Nature 391, 298–301 (1998)
Price, E. R. et al. Lineage-specific signalling in melanocytes. C-kit stimulation recruits p300/CBP to microphthalmia. J. Biol. Chem. 273, 17983–17986 (1998)
Wu, M. et al. c-Kit triggers dual phosphorylations, which couple activation and degradation of the essential melanocyte factor Mi. Genes Dev. 14, 301–312 (2000)
Loercher, A. E., Tank, E. M., Delston, R. B. & Harbour, J. W. MITF links differentiation with cell cycle arrest in melanocytes by transcriptional activation of INK4A. J. Cell Biol. 168, 35–40 (2005)
Carreira, S. et al. Mitf cooperates with Rb1 and activates p21Cip1 expression to regulate cell cycle progression. Nature 433, 764–769 (2005)
Davies, H. et al. Mutations of the BRAF gene in human cancer. Nature 417, 949–954 (2002)
Jafari, M. et al. Analysis of ras mutations in human melanocytic lesions: activation of the ras gene seems to be associated with the nodular type of human malignant melanoma. J. Cancer Res. Clin. Oncol. 121, 23–30 (1995)
Kubo, A. et al. The p16 status of tumour cell lines identifies small molecule inhibitors specific for cyclin-dependent kinase 4. Clin. Cancer Res. 5, 4279–4286 (1999)
McGill, G. G. et al. Bcl2 regulation by the melanocyte master regulator Mitf modulates lineage survival and melanoma cell viability. Cell 109, 707–718 (2002)
Du, J. et al. Critical role of CDK2 for melanoma growth linked to its melanocyte-specific transcriptional regulation by MITF. Cancer Cell 6, 565–576 (2004)
Chu, W. et al. Tyrosinase-related protein 2 as a mediator of melanoma specific resistance to cis-diamminedichloroplatinum(II): therapeutic implications. Oncogene 19, 395–402 (2000)
Berger, R. et al. Androgen-induced differentiation and tumorigenicity of human prostate epithelial cells. Cancer Res. 64, 8867–8875 (2004)
Chen, C. D. et al. Molecular determinants of resistance to antiandrogen therapy. Nature Med. 10, 33–39 (2004)
Berger, A. J. et al. Automated quantitative analysis (AQUA) of HDM2 expression in malignant melanoma shows association with early stage disease and improved outcome. Cancer Res. (in the press)
Rubin, M. A. et al. Overexpression, amplification, and androgen regulation of TPD52 in prostate cancer. Cancer Res. 64, 3814–3822 (2004)
Acknowledgements
We thank D. Scudiero and R. Camalier for provision of NCI60 cell lines and DNAs, O. Kabbarah and L. Chin for discussions and provision of reagents, F. Chen and C. Ladd-Acosta for excellent technical assistance, L. Ziaugra and S. Gabriel for assistance with the BRAF(V600E) genotyping assay, and M. Loda for expert advice. This work was supported by grants from the National Institutes of Health (L.A.G., M.A.R., D.L.R. and D.E.F.), the Swedish Wenner-Gren Foundation (H.R.W.), the Center of Molecular Medicine, Austrian Academy of Sciences (S.N.W.), the Howard Hughes Medical Institute (T.R.G.), the American Cancer Society (M.L.M.), the Flight Attendant Medical Research Institute (M.L.M.), the Doris Duke Foundation (D.E.F.), the Tisch Family Foundation (W.R.S.), and the Damon Runyon Cancer Research Foundation (W.R.S.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The GEO accession number is GSE2520. Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
Supplementary information
Supplementary Figure S1
Full cluster-ordered data set of NCI60 samples and SNPs (derived from CentXba™ array data). (PDF 63 kb)
Supplementary Figure S2
Melanoma tissue array clinical parameters-I: age, gender, and anatomic location of tumour. (PDF 85 kb)
Supplementary Figure S3
Melanoma tissue array clinical parameters -II: Clark level, Breslow depth, and immune response. (PDF 642 kb)
Supplementary Figure S4
Expression of dominant-negative MITF following adenoviral infection. (PDF 34 kb)
Supplementary Figure S5
Pharmacologic analysis of NCI60 cell lines with and without copy gain at the MITF (3p) locus. (PDF 68 kb)
Supplementary Figure Legends
Legends to accompany Supplementary Figures. (DOC 31 kb)
Supplementary Methods
This file contains additional SNP array descriptions, methods, and references. (DOC 83 kb)
Supplementary Table S1
Primer sequences used for quantitative and allele-specific PCR. (DOC 24 kb)
Supplementary Table S2
3p14 dosage, BRAF(V600E) mutation, and CDKN2A (p16) status in NCI60 melanoma cell lines. (DOC 26 kb)
Supplementary Table S3
MITF copy number distribution on the melanoma tissue microarray. (DOC 22 kb)
Supplementary Notes
MIAME Checklist (DOC 73 kb)
Rights and permissions
About this article
Cite this article
Garraway, L., Widlund, H., Rubin, M. et al. Integrative genomic analyses identify MITF as a lineage survival oncogene amplified in malignant melanoma. Nature 436, 117–122 (2005). https://doi.org/10.1038/nature03664
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature03664
This article is cited by
-
Whole-genome resequencing of Chinese indigenous sheep provides insight into the genetic basis underlying climate adaptation
Genetics Selection Evolution (2024)
-
Dormancy of cutaneous melanoma
Cancer Cell International (2024)
-
Rapidly Evolving Pre- and Post-surgical Systemic Treatment of Melanoma
American Journal of Clinical Dermatology (2024)
-
Identification of BRAF, CCND1, and MYC mutations in a patient with multiple primary malignant tumors: a case report and review of the literature
World Journal of Surgical Oncology (2023)
-
GATA2 co-opts TGFβ1/SMAD4 oncogenic signaling and inherited variants at 6q22 to modulate prostate cancer progression
Journal of Experimental & Clinical Cancer Research (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.