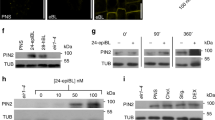
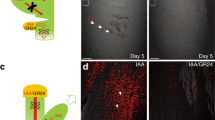
Abstract
One of the mechanisms by which signalling molecules regulate cellular behaviour is modulating subcellular protein translocation. This mode of regulation is often based on specialized vesicle trafficking, termed constitutive cycling, which consists of repeated internalization and recycling of proteins to and from the plasma membrane1. No such mechanism of hormone action has been shown in plants although several proteins, including the PIN auxin efflux facilitators, exhibit constitutive cycling2,3. Here we show that a major regulator of plant development, auxin, inhibits endocytosis. This effect is specific to biologically active auxins and requires activity of the Calossin-like protein BIG. By inhibiting the internalization step of PIN constitutive cycling, auxin increases levels of PINs at the plasma membrane. Concomitantly, auxin promotes its own efflux from cells by a vesicle-trafficking-dependent mechanism. Furthermore, asymmetric auxin translocation during gravitropism is correlated with decreased PIN internalization. Our data imply a previously undescribed mode of plant hormone action: by modulating PIN protein trafficking, auxin regulates PIN abundance and activity at the cell surface, providing a mechanism for the feedback regulation of auxin transport.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Royle, S. & Murrell-Lagnado, R. Constitutive cycling: a general mechanism to regulate cell surface proteins. BioEssays 25, 39–46 (2003)
Geldner, N., Friml, J., Stierhof, Y.-D., Jürgens, G. & Palme, K. Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature 413, 425–428 (2001)
Geldner, N. et al. The Arabidopsis GNOM ARF-GEF mediates endosomal re-cycling, auxin transport, and auxin-dependent plant growth. Cell 112, 219–230 (2003)
Friml, J., Wisniewska, J., Benková, E., Mendgen, K. & Palme, K. Lateral relocation of auxin efflux regulator AtPIN3 mediates tropism in Arabidopsis. Nature 415, 806–809 (2002)
Friml, J. et al. Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature 426, 147–153 (2003)
Benková, E. et al. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115, 591–602 (2003)
Gray, W. R., Kepinski, S., Rouse, D., Leyser, O. & Estelle, M. Auxin regulates SCFTIR1-dependent degradation of AUX/IAA proteins. Nature 414, 271–276 (2001)
Friml, J. Auxin transport—shaping the plant. Curr. Opin. Plant Biol. 6, 7–12 (2003)
Baluška, F. et al. F-actin-dependent endocytosis of cell wall pectins in meristematic root cells. Insights from brefeldin A-induced compartments. Plant Physiol. 130, 422–431 (2002)
Von Gadow, A., Joubert, E. & Hansmann, C. F. Comparison of the antioxidant activity of aspalathin with that of other plant phenols of rooibos tea (Aspalathus linearis), alpha-tocopherol, BHT, and BHA. J. Agric. Food Chem. 45, 632–638 (1997)
Karlin-Neumann, G., Brusslan, J. & Tobin, E. Phytochrome control of the tms2 gene in transgenic Arabidopsis: a strategy for selecting mutants in the signal transduction pathway. Plant Cell 3, 573–582 (1991)
Boerjan, W. et al. Superroot, a recessive mutation in Arabidopsis, confers auxin overproduction. Plant Cell 7, 1405–1419 (1995)
Zhao, Y. et al. A role for flavin monooxygenase-like enzymes in auxin biosynthesis. Science 291, 306–309 (2001)
Swarup, R. et al. Localisation of the auxin permease AUX1 suggests two functionally distinct hormone transport pathways operate in the Arabidopsis root apex. Genes Dev. 15, 2648–2653 (2001)
Xu, J. & Scheres, B. Dissection of Arabidopsis ADP-RIBOSYLATION FACTOR 1 function in epidermal cell polarity. Plant Cell 17, 525–536 (2005)
Emans, N., Zimmermann, S. & Fischer, R. Uptake of a fluorescent marker in plant cells is sensitive to brefeldin A and wortmannin. Plant Cell 14, 71–86 (2002)
Delbarre, A., Muller, P., Imhoff, V. & Guern, J. Comparison of mechanisms controlling uptake and accumulation of 2,4-dichlorophenoxy acetic acid, naphthalene-1-acetic acid, and indole-3-acetic acid in suspension-cultured tobacco cells. Planta 198, 532–541 (1996)
Ruegger, M. et al. Reduced naphthylphthalamic acid binding in the tir3 mutant of Arabidopsis is associated with a reduction in polar auxin transport and diverse morphological defects. Plant Cell 9, 745–757 (1997)
Gil, P. et al. BIG: a calossin-like protein required for polar auxin transport in Arabidopsis. Genes Dev. 15, 1985–1997 (2001)
Kanyuka, K. et al. Mutations in the huge Arabidopsis gene BIG affect a range of hormone and light responses. Plant J. 35, 57–70 (2003)
Berleth, T. & Sachs, T. Plant morphogenesis: long-distance coordination and local patterning. Curr. Opin. Plant Biol. 4, 57–62 (2001)
Morris, D. A. Transmembrane auxin carrier systems—dynamic regulators of polar auxin transport. Plant Growth Regul. 32, 161–172 (2000)
Petrášek, J., Elčkner, M., Morris, D. A. & Zažímalová, E. Auxin efflux carrier activity and auxin accumulation regulate cell division and polarity in tobacco cells. Planta 216, 302–308 (2002)
Petrášek, J. et al. Do phytotropins inhibit auxin efflux by impairing vesicle traffic? Plant Physiol. 131, 254–263 (2003)
Delarue, M., Muller, P., Bellini, C. & Delbarre, A. Increased auxin efflux in the IAA-overproducing sur1 mutant of Arabidopsis thaliana: a mechanism of reducing auxin levels? Physiol. Plant. 107, 120–127 (1999)
Richards, S., Hillman, T. & Stern, M. Mutations in the Drosophila pushover gene confer increased neuronal excitability and spontaneous synaptic vesicle fusion. Genetics 142, 1215–1223 (1996)
Cutler, S., Ehrhardt, D., Griffitts, J. & Somerville, C. Random GFP::cDNA fusions enable visualisation of subcellular structures in cells of Arabidopsis at a high frequency. Proc. Natl Acad. Sci. USA 97, 3718–3723 (2000)
Friml, J., Benková, E., Mayer, U., Palme, K. & Muster, G. Automated whole-mount localization techniques for plant seedlings. Plant J. 34, 115–124 (2003)
Friml, J. et al. AtPIN4 mediates sink-driven auxin gradients and root patterning in Arabidopsis. Cell 108, 661–673 (2002)
Pimpl, P. et al. In situ localization and in vitro induction of plant COPI -coated vesicles. Plant Cell 12, 2219–2236 (2000)
Acknowledgements
We thank C. Bellini, M. Bennett, M. Estelle, M. Grebe, W. Michalke, D. Robinson and Y. Zhao for sharing material, and E. Benková, P. Brewer, J. Eder, J. Malbeck, C. Oecking, M. Sauer, H. Stransky and D. Weijers for technical assistance and discussions. This work was supported by the Volkswagenstiftung (J.F. and T.P.), the F. Ebert Stiftung (J.K.-V.), the Deutsche Forschungsgemeinschaft (N.G., G.J. and Y.-D. S.), the Grant Agency of the Academy of Sciences of the Czech Republic (J.P. and E.Z.) and the Royal Society of London (D.A.M.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
Supplementary information
Supplementary Figure S1
Auxins inhibit BFA-induced internalisation of constitutively cycling PM markers. (JPG 337 kb)
Supplementary Figure S2
Inhibitors of protein expression and degradation confirm BFA-visualized constitutive cycling of PIN1. (JPG 66 kb)
Supplementary Figure S3
Other plant hormones do not inhibit internalisation of constitutively cycling proteins. (JPG 109 kb)
Supplementary Figure S4
5 µM concentrations of NAA and 2,4-D inhibit BFA-induced internalisation of constitutively cycling proteins. (JPG 336 kb)
Supplementary Figure S5
IAA is unstable in Arabidopsis medium but, if stabilised, it inhibits BFA-induced internalisation at 5 µM concentrations. (JPG 178 kb)
Supplementary Figure S6
Auxins down-regulate but do not completely block BFA-induced internalisation of constitutively cycling proteins. (JPG 127 kb)
Supplementary Figure S7
NAA does not influence BFA uptake in Arabidopsis root tissues. (JPG 73 kb)
Supplementary Figure S8
Auxins do not affect the morphology of selected subcellular structures. (JPG 146 kb)
Supplementary Figure S9
Auxins inhibit the BFA-induced internalisation of PM proteins but do not affect BFA-induced aggregation of endosomes. (JPG 160 kb)
Supplementary Figure S10
Auxin efflux in suspension-cultured BY-2 and VBI-0 tobacco cells. (JPG 72 kb)
Rights and permissions
About this article
Cite this article
Paciorek, T., Zažímalová, E., Ruthardt, N. et al. Auxin inhibits endocytosis and promotes its own efflux from cells. Nature 435, 1251–1256 (2005). https://doi.org/10.1038/nature03633
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature03633
This article is cited by
-
3,4-Dichlorophenylacetic acid acts as an auxin analog and induces beneficial effects in various crops
Communications Biology (2024)
-
The Glucose-Related Decrease in Polar Auxin Transport During Ripening and its Possible Role in Grapevine Berry Coloring
Journal of Plant Growth Regulation (2023)
-
Strigolactones inhibit auxin feedback on PIN-dependent auxin transport canalization
Nature Communications (2020)
-
Involvement of Arabidopsis BIG protein in cell death mediated by Myo-inositol homeostasis
Scientific Reports (2020)
-
The BIG gene controls size of shoot apical meristems in Arabidopsis thaliana
Plant Cell Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.