Abstract
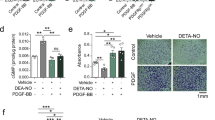
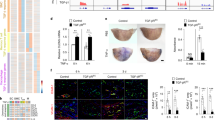
Platelet-derived growth factor (PDGF) is a potent mitogenic and migratory factor that regulates the tyrosine phosphorylation of a variety of signalling proteins via intracellular production of H2O2 (refs 1, 2–3). Mammalian 2-Cys peroxiredoxin type II (Prx II; gene symbol Prdx2) is a cellular peroxidase that eliminates endogenous H2O2 produced in response to growth factors such as PDGF and epidermal growth factor4; however, its involvement in growth factor signalling is largely unknown. Here we show that Prx II is a negative regulator of PDGF signalling. Prx II deficiency results in increased production of H2O2, enhanced activation of PDGF receptor (PDGFR) and phospholipase Cγ1, and subsequently increased cell proliferation and migration in response to PDGF. These responses are suppressed by expression of wild-type Prx II, but not an inactive mutant. Notably, Prx II is recruited to PDGFR upon PDGF stimulation, and suppresses protein tyrosine phosphatase inactivation. Prx II also leads to the suppression of PDGFR activation in primary culture and a murine restenosis model, including PDGF-dependent neointimal thickening of vascular smooth muscle cells. These results demonstrate a localized role for endogenous H2O2 in PDGF signalling, and indicate a biological function of Prx II in cardiovascular disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Heldin, C. H. & Westermark, B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol. Rev. 79, 1283–1316 (1999)
Sundaresan, M., Yu, Z. X., Ferrans, V. J., Irani, K. & Finkel, T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science 270, 296–299 (1995)
Bae, Y. S. et al. Platelet-derived growth factor-induced H2O2 production requires the activation of phosphatidylinositol 3-kinase. J. Biol. Chem. 275, 10527–10531 (2000)
Kang, S. W. et al. Mammalian peroxiredoxin isoforms can reduce hydrogen peroxide generated in response to growth factors and tumor necrosis factor-alpha. J. Biol. Chem. 273, 6297–6302 (1998)
Rhee, S. G., Kang, S. W., Chang, T. S., Jeong, W. & Kim, K. Peroxiredoxin, a novel family of peroxidases. IUBMB Life 52, 35–41 (2001)
Wood, Z. A., Schroder, E., Robin Harris, J. & Poole, L. B. Structure, mechanism and regulation of peroxiredoxins. Trends Biochem. Sci. 28, 32–40 (2003)
Chae, H. Z., Kim, H. J., Kang, S. W. & Rhee, S. G. Characterization of three isoforms of mammalian peroxiredoxin that reduce peroxides in the presence of thioredoxin. Diabetes Res. Clin. Pract. 45, 101–112 (1999)
Kim, H. K. et al. PDGF stimulation of inositol phospholipid hydrolysis requires PLC-γ1 phosphorylation on tyrosine residues 783 and 1254. Cell 65, 435–441 (1991)
Baxter, R. M., Secrist, J. P., Vaillancourt, R. R. & Kazlauskas, A. Full activation of the platelet-derived growth factor β-receptor kinase involves multiple events. J. Biol. Chem. 273, 17050–17055 (1998)
Rhee, S. G. & Bae, Y. S. Regulation of phosphoinositide-specific phospholipase C isozymes. J. Biol. Chem. 272, 15045–15048 (1997)
Sekiya, F., Poulin, B., Kim, Y. J. & Rhee, S. G. Mechanism of tyrosine phosphorylation and activation of phospholipase C-γ1. Tyrosine 783 phosphorylation is not sufficient for lipase activation. J. Biol. Chem. 279, 32181–32190 (2004)
Saito, S. et al. Ligand-independent trans-activation of the platelet-derived growth factor receptor by reactive oxygen species requires protein kinase C-δ and c-Src. J. Biol. Chem. 277, 44695–44700 (2002)
Rosado, J. A. et al. Hydrogen peroxide generation induces pp60src activation in human platelets: evidence for the involvement of this pathway in store-mediated calcium entry. J. Biol. Chem. 279, 1665–1675 (2004)
Yang, K. S. et al. Inactivation of human peroxiredoxin I during catalysis as the result of the oxidation of the catalytic site cysteine to cysteine-sulfinic acid. J. Biol. Chem. 277, 38029–38036 (2002)
Rhee, S. G., Bae, Y. S., Lee, S. R. & Kwon, J. Hydrogen peroxide: a key messenger that modulates protein phosphorylation through cysteine oxidation. Sci STKE 2000, PE1 (2000).
Ross, R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 362, 801–809 (1993)
Ferns, G. A. et al. Inhibition of neointimal smooth muscle accumulation after angioplasty by an antibody to PDGF. Science 253, 1129–1132 (1991)
Persson, C. et al. Site-selective regulation of platelet-derived growth factor beta receptor tyrosine phosphorylation by T-cell protein tyrosine phosphatase. Mol. Cell. Biol. 24, 2190–2201 (2004)
Lee, T. H. et al. Peroxiredoxin II is essential for sustaining life span of erythrocytes in mice. Blood 101, 5033–5038 (2003)
Kang, S. W. et al. Cytosolic peroxiredoxin attenuates the activation of Jnk and p38 but potentiates that of Erk in Hela cells stimulated with tumor necrosis factor-α. J. Biol. Chem. 279, 2535–2543 (2004)
Ohmi, K. et al. A novel aortic smooth muscle cell line obtained from p53 knock out mice expresses several differentiation characteristics. Biochem. Biophys. Res. Commun. 238, 154–158 (1997)
Schober, A. et al. Stabilization of atherosclerotic plaques by blockade of macrophage migration inhibitory factor after vascular injury in apolipoprotein E-deficient mice. Circulation 109, 380–385 (2004)
Woo, H. A. et al. Reversible oxidation of the active site cysteine of peroxiredoxins to cysteine sulfinic acid. Immunoblot detection with antibodies specific for the hyperoxidized cysteine-containing sequence. J. Biol. Chem. 278, 47361–47364 (2003)
Acknowledgements
We thank J. B. Kwon, E. S. Oh and T. H. Lee for reagents and discussions; J. Kim and I. C. Baines for critically reading the manuscript; G. P. Nolan for pBMN retroviral plasmids; A. Kazlauskas for providing HepG2 cells overexpressing PDGFR-β wild type; and D. J. Lee for immunofluorescence staining. We also thank I. H. Lee, H. I. Park and researchers in Labfrontier Life Science Institute for technical assistance. This work was supported by Korea Science and Engineering Foundation through the Center for Cell Signalling Research in Ewha Womans University and by a grant from the Functional Proteomics Center of the 21st Century Frontier Research Program funded by the Ministry of Science and Technology of the Korean government. M.H.C. is supported by a Brain Korea 21 grant from the Ministry of Education and Human Resources Development.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
Supplementary information
Supplementary Methods
This document contains methods sections: (1) cell proliferation, migration and measurement of inositol phosphates; (2) immunoprecipitation and in vitro receptor tyrosine kinase assay; (3) relevant references. (PDF 23 kb)
Supplementary Figure Legends
This document contains the legends for Supplementary Figs 1-7. (PDF 23 kb)
Supplementary Figure 1
Analysis of epidermal growth factor (EGF) signalling in wt and Prx II(-/-) MEFs (a); suppression by Prx II of MEF migration (b). (PDF 221 kb)
Supplementary Figure 2
Catalase inhibition and glutathione depletion result in the elevation of PDGF-induced H2O2 production. (PDF 19 kb)
Supplementary Figure 3
Specificity of the phospho-specific antibodies against tyrosine residues of PDGFRβ. (PDF 161 kb)
Supplementary Figure 4
Characterization of the commercial anti-phospho-PDGFRβ antibodies. (PDF 224 kb)
Supplementary Figure 5
Immunoblot analysis of PDGFR phosphorylation in H2O2-treated MEFs overexpressing Prx II-wt. (PDF 102 kb)
Supplementary Figure 6
Tyrosine phosphorylation of PDGFR in NIH3T3 cells overexpressing Prx II-wt. (PDF 168 kb)
Supplementary Figure 7
Characterization for mouse PDGF-neutralizing activity of anti-PDGF antibody. (PDF 87 kb)
Rights and permissions
About this article
Cite this article
Choi, M., Lee, I., Kim, G. et al. Regulation of PDGF signalling and vascular remodelling by peroxiredoxin II. Nature 435, 347–353 (2005). https://doi.org/10.1038/nature03587
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature03587
This article is cited by
-
Exploring the role of PRDX4 in the development of uterine corpus endometrial carcinoma
Medical Oncology (2024)
-
Deficiency of peroxiredoxin 2 exacerbates angiotensin II-induced abdominal aortic aneurysm
Experimental & Molecular Medicine (2020)
-
Role of Peroxiredoxins in Protecting Against Cardiovascular and Related Disorders
Cardiovascular Toxicology (2020)
-
A Comparative Review of the Effect of Microcystin-LR on the Proteome
Exposure and Health (2020)
-
Effects of Peroxiredoxin 2 in Neurological Disorders: A Review of its Molecular Mechanisms
Neurochemical Research (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.