Abstract
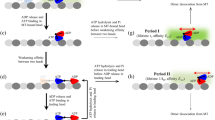
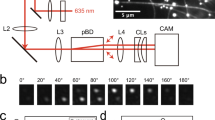
Kinesin is a molecular walking machine that organizes cells by hauling packets of components directionally along microtubules. The physical mechanism that impels directional stepping is uncertain. We show here that, under very high backward loads, the intrinsic directional bias in kinesin stepping can be reversed such that the motor walks sustainedly backwards in a previously undescribed mode of ATP-dependent backward processivity. We find that both forward and backward 8-nm steps occur on the microsecond timescale and that both occur without mechanical substeps on this timescale. The data suggest an underlying mechanism in which, once ATP has bound to the microtubule-attached head, the other head undergoes a diffusional search for its next site, the outcome of which can be biased by an applied load.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kaseda, K., Higuchi, H. & Hirose, K. Alternate fast and slow stepping of a heterodimeric kinesin molecule. Nature Cell Biol. 5, 1079–1082 (2003)
Asbury, C. L., Fehr, A. N. & Block, S. M. Kinesin moves by an asymmetric hand-over-hand mechanism. Science 302, 2130–2134 (2003)
Yildiz, A., Tomishige, M., Vale, R. D. & Selvin, P. R. Kinesin walks hand-over-hand. Science 303, 676–678 (2004)
Svoboda, K., Schmidt, C. F., Schnapp, B. J. & Block, S. M. Direct observation of kinesin stepping by optical trapping interferometry. Nature 365, 721–727 (1993)
Kojima, H., Muto, E., Higuchi, H. & Yanagida, T. Mechanics of single kinesin molecules measured by optical trapping nanometry. Biophys. J. 73, 2012–2022 (1997)
Coppin, C. M., Finer, J. T., Spudich, J. A. & Vale, R. D. Detection of sub-8-nm movements of kinesin by high-resolution optical-trap microscopy. Proc. Natl Acad. Sci. USA 93, 1913–1917 (1996)
Nishiyama, M., Muto, E., Inoue, Y., Yanagida, T. & Higuchi, H. Substeps within the 8-nm step of the ATPase cycle of single kinesin molecules. Nature Cell Biol. 3, 425–428 (2001)
Hua, W., Young, E. C., Fleming, M. L. & Gelles, J. Coupling of kinesin steps to ATP hydrolysis. Nature 388, 390–393 (1997)
Schnitzer, M. J. & Block, S. M. Kinesin hydrolyses one ATP per 8-nm step. Nature 388, 386–390 (1997)
Coy, D. L., Wagenbach, M. & Howard, J. Kinesin takes one 8-nm step for each ATP that it hydrolyzes. J. Biol. Chem. 274, 3667–3671 (1999)
Visscher, K., Schnitzer, M. J. & Block, S. M. Single kinesin molecules studied with a molecular force clamp. Nature 400, 184–189 (1999)
Hackney, D. D. Evidence for alternating head catalysis by kinesin during microtubule-stimulated ATP hydrolysis. Proc. Natl Acad. Sci. USA 91, 6865–6869 (1994)
Cross, R. A. The kinetic mechanism of kinesin. Trends Biochem. Sci. 29, 301–309 (2004)
Tsiavaliaris, G., Fujita-Becker, S. & Manstein, D. J. Molecular engineering of a backwards-moving myosin motor. Nature 427, 558–561 (2004)
Reconditi, M. et al. The myosin motor in muscle generates a smaller and slower working stroke at higher load. Nature 428, 578–581 (2004)
Rice, S. et al. A structural change in the kinesin motor protein that drives motility. Nature 402, 778–784 (1999)
Nishiyama, M., Higuchi, H. & Yanagida, T. Chemomechanical coupling of the forward and backward steps of single kinesin molecules. Nature Cell Biol. 4, 790–797 (2002)
Okada, Y., Higuchi, H. & Hirokawa, N. Processivity of the single-headed kinesin KIF1A through biased binding to tubulin. Nature 424, 574–577 (2003)
Block, S. M., Goldstein, L. S. & Schnapp, B. J. Bead movement by single kinesin molecules studied with optical tweezers. Nature 348, 348–352 (1990)
Hunt, A. J., Gittes, F. & Howard, J. The force exerted by a single kinesin molecule against a viscous load. Biophys. J. 67, 766–781 (1994)
Svoboda, K. & Block, S. M. Force and velocity measured for single kinesin molecules. Cell 77, 773–784 (1994)
Kawaguchi, K. & Ishiwata, S. Temperature dependence of force, velocity, and processivity of single kinesin molecules. Biochem. Biophys. Res. Commun. 272, 895–899 (2000)
Kramers, H. A. Brownian motion in a field of force and the diffusion limit of chemical reactions. Physica 7, 284–304 (1940)
Nishiyama, M., Higuchi, H., Ishii, Y., Taniguchi, Y. & Yanagida, T. Single molecule processes on the stepwise movement of ATP-driven molecular motors. Biosystems 71, 145–156 (2003)
Howard, J. Mechanics of Motor Proteins and the Cytoskeleton (Sinauer, Sunderland, Massachusetts, 2001)
Coppin, C. M., Pierce, D. W., Hsu, L. & Vale, R. D. The load dependence of kinesin's mechanical cycle. Proc. Natl Acad. Sci. USA 94, 8539–8544 (1997)
Hirose, K., Lockhart, A., Cross, R. A. & Amos, L. A. Three-dimensional cryoelectron microscopy of dimeric kinesin and ncd motor domains on microtubules. Proc. Natl Acad. Sci. USA 93, 9539–9544 (1996)
Schief, W. R. & Howard, J. Conformational changes during kinesin motility. Curr. Opin. Cell Biol. 13, 19–28 (2001)
Rice, S. et al. Thermodynamic properties of the Kinesin neck-region docking to the catalytic core. Biophys. J. 84, 1844–1854 (2003)
Schnitzer, M. J., Visscher, K. & Block, S. M. Force production by single kinesin motors. Nature Cell Biol. 2, 718–723 (2000)
Fisher, M. E. & Kolomeisky, A. B. Simple mechanochemistry describes the dynamics of kinesin molecules. Proc. Natl Acad. Sci. USA 98, 7748–7753 (2001)
Thomas, N., Imafuku, Y., Kamiya, T. & Tawada, K. Kinesin: a molecular motor with a spring in its step. Proc. R. Soc. Lond. B 269, 2363–2371 (2002)
Block, S. M., Asbury, C. L., Shaevitz, J. W. & Lang, M. J. Probing the kinesin reaction cycle with a 2D optical force clamp. Proc. Natl Acad. Sci. USA 100, 2351–2356 (2003)
Huxley, A. F. Muscle structure and theories of contraction. Prog. Biophys. Biophys. Chem. 7, 257–318 (1957)
Huxley, A. F. & Simmons, R. M. Proposed mechanism of force generation in striated muscle. Nature 233, 533–538 (1971)
Acknowledgements
We thank J. Howard for the gift of the Drososphila kinesin, the reviewers of this manuscript for careful and constructive criticism, and Marie Curie Cancer Care for unswerving support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
Supplementary information
Supplementary Figure S1
Simulated steps added to bead position noise. Testing the detection limits for steps by adding various simulated steps to real measured noise and trying to detect the steps using our algorithm. (PDF 2001 kb)
Supplementary Figure S2
Dwell time distributions at various loads and ATP concentrations. (PDF 764 kb)
Supplementary Figure Legends
Legends to accompany Supplementary Figures S1 and S2. (DOC 21 kb)
Rights and permissions
About this article
Cite this article
Carter, N., Cross, R. Mechanics of the kinesin step. Nature 435, 308–312 (2005). https://doi.org/10.1038/nature03528
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature03528
This article is cited by
-
Super-resolved FRET and co-tracking in pMINFLUX
Nature Photonics (2024)
-
A DNA turbine powered by a transmembrane potential across a nanopore
Nature Nanotechnology (2023)
-
High-performance Marangoni hydrogel rotors with asymmetric porosity and drag reduction profile
Nature Communications (2023)
-
The reaction-diffusion basis of animated patterns in eukaryotic flagella
Nature Communications (2023)
-
Controlling dynamics in extended molecular frameworks
Nature Reviews Chemistry (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.