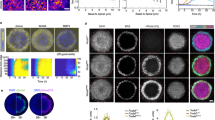
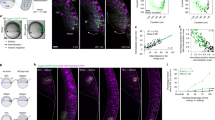
Abstract
The precise specification of left–right asymmetry is an essential process for patterning internal organs in vertebrates. In mouse embryonic development, the symmetry-breaking process in left–right determination is initiated by a leftward extraembryonic fluid flow on the surface of the ventral node. However, it is not known whether the signal transduction mechanism of this flow is chemical or mechanical. Here we show that fibroblast growth factor (FGF) signalling triggers secretion of membrane-sheathed objects 0.3–5 µm in diameter termed ‘nodal vesicular parcels’ (NVPs) that carry Sonic hedgehog and retinoic acid. These NVPs are transported leftward by the fluid flow and eventually fragment close to the left wall of the ventral node. The silencing effects of the FGF-receptor inhibitor SU5402 on NVP secretion and on a downstream rise in Ca2+ were sufficiently reversed by exogenous Sonic hedgehog peptide or retinoic acid, suggesting that FGF-triggered surface accumulation of cargo morphogens may be essential for launching NVPs. Thus, we propose that NVP flow is a new mode of extracellular transport that forms a left–right gradient of morphogens.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hirokawa, N. Stirring up development with the heterotrimeric kinesin KIF3. Traffic 1, 29–34 (2000)
Nonaka, S. et al. Randomization of left–right asymmetry due to loss of nodal cilia generating leftward flow of extra embryonic fluid in mice lacking KIF3B motor protein. Cell 95, 829–837 (1998)
Takeda, S. et al. Left-right asymmetry and kinesin superfamily protein KIF3A: new insights in determination of laterality and mesoderm induction by kif3A-/- mice analysis. J. Cell Biol. 145, 825–836 (1999)
Okada, Y. et al. Abnormal nodal flow precedes situs inversus in iv and inv mice. Mol. Cell 4, 459–468 (1999)
Nonaka, S., Shiratori, H., Saijoh, Y. & Hamada, H. Determination of left-right patterning of the mouse embryo by artificial nodal flow. Nature 418, 96–99 (2002)
Afzelius, B. A. Cilia-related diseases. J. Pathol. 204, 470–477 (2004)
Tabin, C. J. & Vogan, K. J. A two-cilia model for vertebrate left-right axis specification. Genes Dev. 17, 1–6 (2003)
Yokoyama, T. Motor or sensor: A new aspect of primary cilia function. Anat. Sci. Int. 79, 47–54 (2004)
McGrath, J., Somlo, S., Makova, S., Tian, X. & Brueckner, M. Two populations of node monocilia initiate left-right asymmetry in the mouse. Cell 114, 61–73 (2003)
Dubrulle, J., McGrew, M. J. & Pourquie, O. FGF signalling controls somite boundary position and regulates segmentation clock control of spatiotemporal Hox gene activation. Cell 106, 219–232 (2001)
Dubrulle, J. & Pourquie, O. fgf8 mRNA decay establishes a gradient that couples axial elongation to patterning in the vertebrate embryo. Nature 427, 419–422 (2004)
Meyers, E. N. & Martin, G. R. Differences in left-right axis pathways in mouse and chick: functions of FGF8 and SHH. Science 285, 403–406 (1999)
Kondo, S. et al. KIF3A is a new microtubule-based anterograde motor in the nerve axon. J. Cell Biol. 125, 1095–1107 (1994)
Rosenbaum, J. L. & Witman, G. B. Intraflagellar transport. Nature Rev. Mol. Cell Biol. 3, 813–825 (2002)
Scholey, J. M. Intraflagellar transport. Annu. Rev. Cell Dev. Biol. 19, 423–443 (2003)
Mohammadi, M. et al. Structures of the tyrosine kinase domain of fibroblast growth factor receptor in complex with inhibitors. Science 276, 955–960 (1997)
Kos, F. J. & Chin, C. S. Costimulation of T cell receptor-triggered IL-2 production by Jurkat T cells via fibroblast growth factor receptor 1 upon its engagement by CD56. Immunol. Cell Biol. 80, 364–369 (2002)
Tsukui, T. et al. Multiple left-right asymmetry defects in Shh-/- mutant mice unveil a convergence of the Shh and retinoic acid pathways in the control of Lefty-1. Proc. Natl Acad. Sci. USA 96, 11376–11381 (1999)
Zhang, X. M., Ramelho-Santos, M. & McMahon, A. P. Smoothened mutants reveal redundant roles for Shh and Ihh signaling including regulation of L/R asymmetry by the mouse node. Cell 105, 781–792 (2001)
Raya, Á. et al. Notch activity acts as a sensor for extracellular calcium during vertebrate left-right determination. Nature 427, 121–128 (2004)
Mathieu, J. et al. Nodal and Fgf pathways interact through a positive regulatory loop and synergize to maintain mesodermal cell populations. Development 131, 629–641 (2004)
Kawakami, T. et al. Mouse dispatched mutants fail to distribute hedgehog proteins and are defective in hedgehog signaling. Development 129, 5753–5765 (2002)
Ye, W., Shimamura, K., Rubenstein, J. L. R., Hynes, M. A. & Rosenthal, A. FGF and Shh signals control dopaminergic and serotonergic cell fate in the anterior neural plate. Cell 93, 755–766 (1998)
Kessaris, N., Jamen, F., Rubin, L. L. & Richardson, W. D. Cooperation between sonic hedgehog and fibroblast growth factor/MAPK signalling pathways in neocortical precursors. Development 131, 1289–1298 (2004)
Scherz, P. J., Harfe, B. D., McMahon, A. P. & Tabin, C. J. The limb bud Shh-Fgf feedback loop is terminated by expansion of former ZPA cells. Science 305, 396–399 (2004)
Rice, R. et al. Disruption of Fgf10/Fgfr2b-coordinated epithelial-mesenchymal interactions causes cleft palate. J. Clin. Invest. 113, 1692–1700 (2004)
Zeng, X. et al. A freely diffusible form of Sonic hedgehog mediates long-range signalling. Nature 411, 716–720 (2001)
Huangfu, D. et al. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature 426, 83–87 (2003)
Ramírez-Weber, F.-A. & Kornberg, T. B. Cytonemes: cellular processes that project to the principal signalling center in Drosophila imaginal discs. Cell 97, 599–607 (1999)
Sato, M. & Kornberg, T. B. FGF is an essential mitogen and chemoattractant for the air sacs of the Drosophila tracheal system. Dev. Cell 3, 195–207 (2002)
Sturm, K. & Tam, P. P. L. Isolation and culture of whole postimplantation embryos and germ layer derivatives. Methods Enzymol. 225, 164–190 (1993)
Tanaka, Y., Kawahata, K., Nakata, T. & Hirokawa, N. Chronological expression of microtubule-associated proteins (MAPs) in EC cell P19 after neuronal induction by retinoic acid. Brain Res. 596, 269–278 (1992)
Acknowledgements
We thank P. Tam and colleagues at CMRI for advice on performing embryo dissection and whole-embryo culture. We also thank S. Takeda, R. Takemura, J. Teng, Y. Noda, H. Sato, N. Onouchi, H. Fukuda, M. Sugaya, T. Akamatsu, T. Aizawa and others from the Hirokawa laboratory for providing materials, discussions and technical assistance.This study has been supported by a Center of Excellence Grant-in-Aid (to N.H.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.Author contributions Y.O. helped to produce Fig. 3.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
Supplementary Movie S1
Leftward nodal flow of fluorescent beads irrespective of FGF signalling. (MOV 1285 kb)
Supplementary Movie S2
Fluorescent images of mouse nodes in ventral views with unidirectional flow of NVPs towards the left. (MOV 822 kb)
Supplementary Movie S3
Ventral view of a mouse node, whose NVP flow is suppressed by 20 µM SU5402. (MOV 940 kb)
Supplementary Movie S4
Ball-throwing release of an NVP from the tip of a dynamically protruding microvillum of the right wall of a node. (MOV 641 kb)
Supplementary Movie S5
Crawling and smashed appearance of NVPs. (MOV 481 kb)
Supplementary Movie S6
Fragmentation of an NVP in the proximity of the left wall. (MOV 111 kb)
Supplementary Movie S7
Restored NVP flow by the addition of SHH-N peptide in the presence of SU5402. (MOV 2932 kb)
Supplementary Movie S8
NVP flow is restored by the addition of 10-7M RA in the presence of SU5402. (MOV 1643 kb)
Supplementary Movie S9
Ventral view of a node treated with SU5402 and IHH-N peptide. (MOV 1057 kb)
Supplementary Movie S10
Ventral view of an iv/iv mutant node. (MOV 2700 kb)
Supplementary Movie S11
Ventral view of a kif3a-/- mutant node lacking the nodal cilia. (MOV 2932 kb)
Supplementary Movie 12
Rotating view of a mouse ventral node fluorescently labelled by 5E1 antibody against SHH-N. (MOV 551 kb)
Rights and permissions
About this article
Cite this article
Tanaka, Y., Okada, Y. & Hirokawa, N. FGF-induced vesicular release of Sonic hedgehog and retinoic acid in leftward nodal flow is critical for left–right determination. Nature 435, 172–177 (2005). https://doi.org/10.1038/nature03494
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature03494
This article is cited by
-
Versatile extracellular vesicle-mediated information transfer: intercellular synchronization of differentiation and of cellular phenotypes, and future perspectives
Inflammation and Regeneration (2024)
-
Non-canonical non-genomic morphogen signaling in anucleate platelets: a critical determinant of prothrombotic function in circulation
Cell Communication and Signaling (2024)
-
R-Spondin 2 governs Xenopus left-right body axis formation by establishing an FGF signaling gradient
Nature Communications (2024)
-
Single cell RNA analysis of the left–right organizer transcriptome reveals potential novel heterotaxy genes
Scientific Reports (2023)
-
A change of heart: new roles for cilia in cardiac development and disease
Nature Reviews Cardiology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.