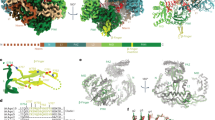
Abstract
RNA interference and related RNA silencing phenomena use short antisense guide RNA molecules to repress the expression of target genes1,2. Argonaute proteins3, containing amino-terminal PAZ (for PIWI/Argonaute/Zwille) domains and carboxy-terminal PIWI domains, are core components of these mechanisms. Here we show the crystal structure of a Piwi protein from Archaeoglobus fulgidus (AfPiwi) in complex with a small interfering RNA (siRNA)-like duplex, which mimics the 5′ end of a guide RNA strand bound to an overhanging target messenger RNA. The structure contains a highly conserved metal-binding site that anchors the 5′ nucleotide of the guide RNA. The first base pair of the duplex is unwound, separating the 5′ nucleotide of the guide from the complementary nucleotide on the target strand, which exits with the 3′ overhang through a short channel. The remaining base-paired nucleotides assume an A-form helix, accommodated within a channel in the PIWI domain, which can be extended to place the scissile phosphate of the target strand adjacent to the putative slicer catalytic site. This study provides insights into mechanisms of target mRNA recognition and cleavage by an Argonaute–siRNA guide complex.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Mello, C. C. & Conte, D. Jr Revealing the world of RNA interference. Nature 431, 338–342 (2004)
Meister, G. & Tuschl, T. Mechanisms of gene silencing by double-stranded RNA. Nature 431, 343–349 (2004)
Carmell, M. A., Xuan, Z., Zhang, M. Q. & Hannon, G. J. The Argonaute family: tentacles that reach into RNAi, developmental control, stem cell maintenance, and tumorigenesis. Genes Dev. 16, 2733–2742 (2002)
Hammond, S. M., Boettcher, S., Caudy, A. A., Kobayashi, R. & Hannon, G. J. Argonaute2, a link between genetic and biochemical analyses of RNAi. Science 293, 1146–1150 (2001)
Verdel, A. et al. RNAi-mediated targeting of heterochromatin by the RITS complex. Science 303, 672–676 (2004)
Sontheimer, E. J. Assembly and function of RNA silencing complexes. Nature Rev. Mol. Cell Biol. 6, 127–138 (2005)
Yan, K. S. et al. Structure and conserved RNA binding of the PAZ domain. Nature 426, 468–474 (2003)
Song, J. J. et al. The crystal structure of the Argonaute2 PAZ domain reveals an RNA binding motif in RNAi effector complexes. Nature Struct. Biol. 10, 1026–1032 (2003)
Lingel, A., Simon, B., Izaurralde, E. & Sattler, M. Nucleic acid 3′-end recognition by the Argonaute2 PAZ domain. Nature Struct. Mol. Biol. 11, 576–577 (2004)
Ma, J. B., Ye, K. & Patel, D. J. Structural basis for overhang-specific small interfering RNA recognition by the PAZ domain. Nature 429, 318–322 (2004)
Song, J. J., Smith, S. K., Hannon, G. J. & Joshua-Tor, L. Crystal structure of Argonaute and its implications for RISC slicer activity. Science 305, 1434–1437 (2004)
Parker, J. S., Roe, S. M. & Barford, D. Crystal structure of a PIWI protein suggests mechanisms for siRNA recognition and slicer activity. EMBO J. 23, 4727–4737 (2004)
Liu, J. et al. Argonaute2 is the catalytic engine of mammalian RNAi. Science 305, 1437–1441 (2004)
Nykanen, A., Haley, B. & Zamore, P. D. ATP requirements and small interfering RNA structure in the RNA interference pathway. Cell 107, 309–321 (2001)
Chiu, Y. L. & Rana, T. M. RNAi in human cells: basic structural and functional features of small interfering RNA. Mol. Cell 10, 549–561 (2002)
Martinez, J., Patkaniowska, A., Urlaub, H., Luhrmann, R. & Tuschl, T. Single-stranded antisense siRNAs guide target RNA cleavage in RNAi. Cell 110, 563–574 (2002)
Elbashir, S. M., Martinez, J., Patkaniowska, A., Lendeckel, W. & Tuschl, T. Functional anatomy of siRNAs for mediating efficient RNAi in Drosophila melanogaster embryo lysate. EMBO J. 20, 6877–6888 (2001)
Elbashir, S. M., Lendeckel, W. & Tuschl, T. RNA interference is mediated by 21- and 22-nucleotide RNAs. Genes Dev. 15, 188–200 (2001)
Haley, B. & Zamore, P. D. Kinetic analysis of the RNAi enzyme complex. Nature Struct. Mol. Biol. 11, 599–606 (2004)
Hutvagner, G. & Zamore, P. D. A microRNA in a multiple-turnover RNAi enzyme complex. Science 297, 2056–2060 (2002)
Lewis, B. P., Shih, I. H., Jones-Rhoades, M. W., Bartel, D. P. & Burge, C. B. Prediction of mammalian microRNA targets. Cell 115, 787–798 (2003)
Stark, A., Brennecke, J., Russell, R. B. & Cohen, S. M. Identification of Drosophila MicroRNA targets. PLoS Biol. 1, 397–409 (2003)
Doench, J. G. & Sharp, P. A. Specificity of microRNA target selection in translational repression. Genes Dev. 18, 504–511 (2004)
Lewis, B. P., Burge, C. B. & Bartel, D. P. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are MicroRNA targets. Cell 120, 15–20 (2005)
Khvorova, A., Reynolds, A. & Jayasena, S. D. Functional siRNAs and miRNAs exhibit strand bias. Cell 115, 209–216 (2003)
Schwarz, D. S. et al. Asymmetry in the assembly of the RNAi enzyme complex. Cell 115, 199–208 (2003)
Okamura, K., Ishizuka, A., Siomi, H. & Siomi, M. C. Distinct roles for Argonaute proteins in small RNA-directed RNA cleavage pathways. Genes Dev. 18, 1655–1666 (2004)
Tomari, Y., Matranga, C., Haley, B., Martinez, N. & Zamore, P. D. A protein sensor for siRNA asymmetry. Science 306, 1377–1380 (2004)
Collaborative Computational Project No. 4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D 50, 760–763 (1994)
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D 60, 2126–2132 (2004)
Ma, J.-B. et al. Structural basis for 5′-end-specific recognition of guide RNA by the A. fulgidus Piwi protein. Nature doi:10.1038/nature03514 (this issue)
Acknowledgements
We are grateful to staff at ESRF for help with data collection.The work was funded by Cancer Research–UK, the Wellcome Trust and the Institute of Cancer Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
Supplementary Figure S1
Shows the conformation of the siRNA duplex in the two copies of the AfPiwi-RNA complex per P1 unit cell (a), and a superimposition of the siRNA duplex onto an A-form RNA helix (b). (PPT 363 kb)
Supplementary Figure S2
Shows a multiple sequence alignment of the PIWI domains of AfPiwi, PfAgo and selected eukaryotic Argonautes. (PPT 1405 kb)
Supplementary Figure S3
Shows a superimposition of AfPiwi with the target RNA strand and PfAgo in the region of the putative slicer catalytic site. (PPT 639 kb)
Supplementary Table S1
Table of crystallographic data collection, processing and refinement statistics. (DOC 22 kb)
Rights and permissions
About this article
Cite this article
Parker, J., Roe, S. & Barford, D. Structural insights into mRNA recognition from a PIWI domain–siRNA guide complex. Nature 434, 663–666 (2005). https://doi.org/10.1038/nature03462
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature03462
This article is cited by
-
Structural basis for sequence-specific recognition of guide and target strands by the Archaeoglobus fulgidus Argonaute protein
Scientific Reports (2023)
-
Origins and diversification of animal innate immune responses against viral infections
Nature Ecology & Evolution (2023)
-
An evolutionarily conserved stop codon enrichment at the 5′ ends of mammalian piRNAs
Nature Communications (2022)
-
Binding of guide piRNA triggers methylation of the unstructured N-terminal region of Aub leading to assembly of the piRNA amplification complex
Nature Communications (2021)
-
Prokaryotic Argonaute from Archaeoglobus fulgidus interacts with DNA as a homodimer
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.