Abstract
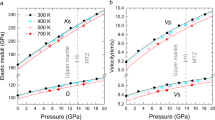
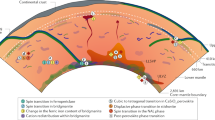
The distribution of water in the Earth's interior reflects the way in which the Earth has evolved, and has an important influence on its material properties. Minerals in the transition zone of the Earth's mantle (from ∼410 to ∼660 km depth) have large water solubility1,2,3, and hence it is thought that the transition zone might act as a water reservoir. When the water content of the transition zone exceeds a critical value, upwelling flow might result in partial melting at ∼410 km, which would affect the distribution of certain elements in the Earth4. However, the amount of water in the transition zone has remained unknown. Here we determined the effects of water and temperature on the electrical conductivity of the minerals wadsleyite and ringwoodite to infer the water content of the transition zone. We find that the electrical conductivity of these minerals depends strongly on water content but only weakly on temperature. By comparing these results with geophysically inferred conductivity5,6,7, we infer that the water content in the mantle transition zone varies regionally, but that its value in the Pacific is estimated to be ∼0.1–0.2 wt%. These values significantly exceed the estimated critical water content in the upper mantle3,8,9, suggesting that partial melting may indeed occur at ∼410 km depth, at least in this region.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Smyth, J. R. β-Mg2SiO4: A potential host for water in the mantle? Am. Mineral. 72, 1051–1055 (1987)
Kawamoto, T., Hervig, R. L. & Holloway, J. R. Experimental evidence for a hydrous transition zone in the early Earth's mantle. Earth Planet. Sci. Lett. 142, 587–592 (1996)
Kohlstedt, D. L., Keppler, H. & Rubie, D. C. The solubility of water in α, β and γ phases of (Mg,Fe)2SiO4 . Contrib. Mineral. Petrol. 123, 345–357 (1996)
Bercovici, D. & Karato, S. Whole-mantle convection and the transition-zone water filter. Nature 425, 39–44 (2003)
Utada, H., Koyama, T., Shimizu, H. & Chave, A. D. A semi-global reference model for electrical conductivity in the mid-mantle beneath the north Pacific region. Geophys. Res. Lett. 30, 1194–1198 (2003)
Ichiki, M. et al. Upper mantle conductivity structure of the back-arc region beneath northeastern China. Geophys. Res. Lett. 28, 3773–3776 (2001)
Tarits, P., Hautot, S. & Perrier, F. Water in the mantle: Results from electrical conductivity beneath the French Alps. Geophys. Res. Lett. 31, L06612, doi:10.1029/2003GL019277 (2004)
Bell, D. R. & Rossman, G. R. Water in Earth's mantle—the role of nominally anhydrous minerals. Science 255, 1391–1397 (1992)
Hirth, G. & Kohlstedt, D. L. Water in oceanic upper mantle—implications for rheology, melt extraction and the evolution of lithosphere. Earth Planet. Sci. Lett. 144, 93–108 (1996)
Kushiro, I. et al. Melting of a peridotite nodule at high pressures and high water pressures. J. Geophys. Res. 73, 6023–6029 (1968)
Hirose, K. & Kushiro, I. Partial melting of dry peridotites at high pressures: Determination of compositions of melts segregated from peridotite using aggregates of diamond. Earth Planet. Sci. Lett. 114, 477–489 (1993)
Grove, T. L. et al. Fractional crystallization and mantle melting controls on calc-alkaline differentiation trends. Contrib. Mineral. Petrol. 145, 515–533 (2003)
Karato, S.,, Paterson, M. S. & Fitz Gerald, J. D. Rheology of synthetic olivine aggregates—influence of grain-size and water. J. Geophys. Res. 91, 8151–8176 (1986)
Mei, S. & Kohlstedt, D. L. Influence of water on plastic deformation of olivine aggregates: 1. Diffusion creep regime. J. Geophys. Res. 105, 21457–21469 (2000)
Karato, S. & Jung, H. Effects of pressure on high-temperature dislocation creep in olivine. Phil. Mag. 83, 401–414 (2003)
Karato, S. The role of hydrogen in the electrical conductivity of the upper mantle. Nature 347, 272–273 (1990)
Farver, J. R. & Yund, R. A. Oxygen fugacity in quartz: dependence on temperature and water fugacity. Chem. Geol. 90, 55–70 (1991)
Karato, S. in Inside the Subduction Factory (ed. Eiler, J.) 138–152 (Am. Geophys. Union, Washington DC, 2003)
Paterson, M. S. The determination of hydroxyl by infrared absorption in quartz, silicate glasses and similar materials. Bull. Mineral. 105, 20–29 (1982)
Xu, Y., Poe, B. T., Shankland, T. J. & Rubie, D. C. Electrical conductivity of olivine, wadsleyite, and ringwoodite under upper-mantle conditions. Science 280, 1415–1418 (1998)
Wood, B. J., Bryndzia, L. T. & Johnson, K. E. Mantle oxidation state and its relationship to tectonic environment and fluid speciation. Science 248, 337–345 (1990)
Xu, Y., Shankland, T. J. & Duba, A. G. Pressure effect on electrical conductivity of mantle olivine. Phys. Earth Planet. Inter. 118, 149–161 (2000)
Acknowledgements
Z. Jiang and Y. Nishihara provided the technical assistance that made this research possible. This work was supported by the NSF of China and the NSF of the United States.Authors' contributions S.-I.K. supervised the whole project. The experimental measurements of electrical conductivity were made by X.H. in collaboration with Y.X., and the theoretical interpretation of the results and the geophysical applications were made by S.-I.K. together with Y.X.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Rights and permissions
About this article
Cite this article
Huang, X., Xu, Y. & Karato, Si. Water content in the transition zone from electrical conductivity of wadsleyite and ringwoodite. Nature 434, 746–749 (2005). https://doi.org/10.1038/nature03426
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature03426
This article is cited by
-
Weak upper-mantle base revealed by postseismic deformation of a deep earthquake
Nature (2023)
-
First-principles investigations of structural, elastic and electronic properties of hydrous fayalite
Arabian Journal of Geosciences (2022)
-
Hydrogen dances in the deep mantle
Nature Geoscience (2021)
-
Quality over quantity: on workflow and model space exploration of 3D inversion of MT data
Earth, Planets and Space (2020)
-
Deep origin of Cenozoic volcanoes in Northeast China revealed by 3-D electrical structure
Science China Earth Sciences (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.