Abstract
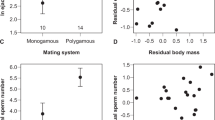
Sperm design and function are important determinants of male reproductive success and are expected to be under strong selection1,2. The way that spermatozoa phenotypes evolve is poorly understood, because there have been few studies of the quantitative genetics of sperm3,4,5. Here we show, in the zebra finch Taeniopygia guttata, an extraordinary degree of inter-male variation in sperm design that is independent of sperm swimming velocity. A quantitative genetics study using data from over 900 zebra finches in a complex breeding experiment showed that sperm head, mid-piece and flagellum length are heritable, that negative genetic correlations exist between sperm traits, and that significant indirect (maternal) genetic effects exist. Selection on the zebra finch sperm phenotype may be low because sperm competition is infrequent in this species6, and this, in combination with negative genetic correlations and maternal genetic effects, may account for the variation in sperm phenotype between males. These results have important implications for the evolution of sperm in other taxa.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Parker, G. A. in Sperm Competition and Sexual Selection (eds Birkhead, T. R. & Møller, A. P.) 3–54 (Academic, London, 1998)
Birkhead, T. R. & Pizzari, T. Post-copulatory sexual selection. Nature Rev. Genet. 3, 262–273 (2002)
Beatty, R. A. The genetics of the mammalian gamete. Biol. Rev. 45, 73–119 (1980)
Morrow, E. H. & Gage, M. J. G. Artificial selection and heritability of sperm length in Gryllus bimaculatus . Heredity 87, 356–362 (2001)
Simmons, L. W. & Kotiaho, J. S. Evolution of ejaculates: patterns of phenotypic and genotypic variation and condition dependence in sperm competition traits. Evolution 56, 1622–1631 (2002)
Birkhead, T. R., Burke, T., Zann, R., Hunter, F. M. & Krupa, A. P. Extra-pair paternity and intraspecific brood parasitism in wild zebra finches Taeniopygia guttata, revealed by DNA fingerprinting. Behav. Ecol. Sociobiol. 27, 315–324 (1990)
Cohen, J. & McNaughton, D. C. Spermatozoa: the probable selection of a small population by the genital tract of the female rabbit. J. Reprod. Fertil. 39, 297–310 (1974)
Birkhead, T. R., Møller, A. P. & Sutherland, W. J. Why do females make it so difficult for males to fertilize their eggs? J. Theor. Biol. 161, 51–60 (1993)
Jamieson, B. G. M. in The Male Gamete: From Basic Science to Clinical Applications (ed. Gagnon, C.) 304–331 (Cache River Press, Vienna, Illinois, 1999)
Cohen, J. Reproduction (Butterworths, London and Boston, 1977)
Morrow, E. H. & Gage, M. J. G. Consistent significant variation between individual males in spermatozoal morphometry. J. Zool. (Lond.) 253, 147–153 (2001)
Kruuk, L. E. B. Estimating genetic parameters in natural populations using the ‘animal model’. Phil. Trans. R. Soc. Lond. B 359, 873–890 (2004)
Boldman, K. G., Kriese, L. A., Van Vleck, L. D., Van Tassell, C. P. & Kachman, S. D. A Manual for Use of MTDFREML. A Set of Programs to Obtain Estimates of Variances and Covariances. Revised United States Department of Agriculture–Agricultural Research Station (US Meat Animal Research Center, Clay Center, Nebraska, 1995)
Birkhead, T. R. & Fletcher, F. Male phenotype and ejaculate quality in the zebra finch Taeniopygia guttata . Proc. R. Soc. Lond. B 262, 329–344 (1995)
Cardullo, R. A. & Baltz, J. M. Metabolic regulation in mammalian sperm: mitochondrial volume determines sperm length and flagellar beat frequency. Cell Motil. Cytoskel. 19, 180–188 (1991)
Froman, D. P. & Feltmann, A. J. Sperm mobility: A quantitative trait in the domestic fowl (Gallus domesticus). Biol. Reprod. 58, 379–384 (1998)
Birkhead, T. R., Martinez, J. G., Burke, T. & Froman, D. P. Sperm mobility determines the outcome of sperm competition in the domestic fowl. Proc. R. Soc. Lond. B 266, 1759–1764 (1999)
Anderson, M. J. & Dixson, A. F. Sperm competition: Motility and the midpiece in primates. Nature 416, 496 (2002)
Birkhead, T. R., Fletcher, F., Pellatt, E. J. & Staples, A. Ejaculate quality and the success of extra-pair copulations in the zebra finch. Nature 377, 422–423 (1995)
Wolf, J. B., Brodie, E. D., Cheverud, J. M., Moore, A. J. & Wade, M. J. Evolutionary consequences of indirect genetic effects. Trends Ecol. Evol. 13, 64–69 (1998)
Frank, S. A. & Hurst, L. D. Mitochondria and male disease. Nature 383, 224 (1996)
Gemmel, N. J., Metcalf, V. J. & Allendorf, F. W. Mother's curse: the effect of mtDNA on individual fitness and population viability. Trends Ecol. Evol. 19, 238–244 (2004)
Zeh, J. Sexy sons: a dead end of cytoplasmic genes. Proc. R. Soc. Lond. B (Suppl.) 271, S306–S309 (2004)
Royle, N. J., Surai, P. F. & Hartley, I. R. The effect of variation in dietary intake on maternal deposition of antioxidants in zebra finch eggs. Funct. Ecol. 17, 472–481 (2003)
Cheverud, J. M., Leamy, L. J., Atchley, W. R. & Rutledge, J. J. Quantitative genetics and the evolution of ontogeny I. Ontogenetic changes in quantitative genetic variance components in randombred mice. Genet. Res. 42, 65–75 (1983)
McFarlane, R. W. The taxonomic significance of avian sperm. Proc. 13th Int. Orn. Congr. 1, 91–102 (1963)
Roff, D. A. The evolution of the G-matrix: selection or drift? Heredity 84, 135–142 (2000)
Zann, R. A. The Zebra Finch: A Synthesis of Field and Laboratory Studies (Oxford Univ. Press, Oxford, 1996)
Lynch, M. & Walsh, B. Genetics and Analysis of Quantitative Traits (Sinauer Associates, Sunderland, Massachusetts, 1998)
Purvis, A. & Rambaut, A. Comparative analysis by independent contrasts (CAIC): an Apple Macintosh application for analysing comparative data. Comput. Appl. Biosci. 11, 247–251 (1995)
Acknowledgements
We are grateful to the following for technical assistance: A. Bamford, H. Basford, S. Bawden, S. Bradish, L. Birkhead, A. Blake, M. Hudson, K. Hutchence, R. Linacre, B. Mappin, A. MacDonald, the late O. Scott-Roberts, D. Rose, J. Shutt, K. Swinglehurst, L. Turton and E. Varsey. We thank F. M. Hunter, I. M. Matthews, N. Roddis and P. Young for help with the project, and A. Beckerman, J. D. Biggins, D. Coltman, J. Cummins, C. Haley, L. Keller, A. Moore, B. Sheldon, J. Slate, J. St John and D. Woolley for advice and/or comments. This study was funded by a NERC research grant to T.R.B.Authors' contributions T.R.B. managed the entire project and wrote the manuscript; E.J.P. managed the birds; P.B. and R.Y. measured the sperm; and H.C.-J. conducted the genetic analyses.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Rights and permissions
About this article
Cite this article
Birkhead, T., Pellatt, E., Brekke, P. et al. Genetic effects on sperm design in the zebra finch. Nature 434, 383–387 (2005). https://doi.org/10.1038/nature03374
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature03374
This article is cited by
-
Reproductive coordination breeds success: the importance of the partnership in avian sperm biology
Behavioral Ecology and Sociobiology (2020)
-
Social dominance explains within-ejaculate variation in sperm design in a passerine bird
BMC Evolutionary Biology (2017)
-
A sex-chromosome inversion causes strong overdominance for sperm traits that affect siring success
Nature Ecology & Evolution (2017)
-
A sex-linked supergene controls sperm morphology and swimming speed in a songbird
Nature Ecology & Evolution (2017)
-
Supergene yields super sperm
Nature Ecology & Evolution (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.